|
1. Chlorobenzene
Cl |
Benzene undergoes a substitution reaction with chlorine only in the presence of halogen carriers. An addition reaction takes place in the absence of a carrier, leading to the formation of benzene hexa — chloride. Iron is the only halogen carrier used extensively in industry, a suitable form being fine iron powder.
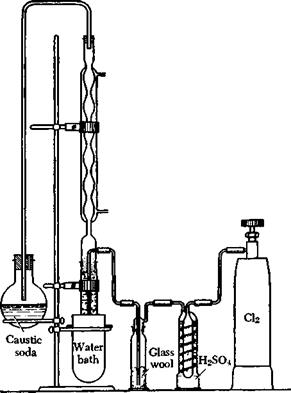
Chlorination is best carried out at about 30°C. At lower temperatures, the reaction proceeds poorly, while at higher temperatures the formation of by-products is favored (di — and polychlorobenzenes, or even benzene hexachloride, especially if the reaction is carried out in sunlight). Chlorine is taken from a cylinder provided with a reduction valve which permits accurate regulation of the gas flow. In all chlorina — tions of this type, it is important to exclude moisture, since even traces of water retard the absoiption of chlorine and favor the formation of by-products. Hence, the reaction flask should be preceded by at least two ordinary gas washing bottles containing concentrated sulfuric acid. More effective still is a so-called “spiral gas washing bottle” in which the gas is made to flow along a long spiral path through the sulfuric acid. The gas washing bottles should be followed by a drying tower filled with pumice, or a “reversed” gas washing bottle filled with glass wool, to catch entrained droplets of sulfuric acid (see Fig. 5).
A relatively tall and narrow glass cylinder may be used advantageously as a chlorination vessel, so that the chlorine is made to pass through a thick layer of the liquid. The vessel is equipped with an efficient reflux condenser, preferably connected by a ground glass joint or, if this is not available, by a cork treated with cellulose acetate varnish. The reaction vessel is placed in a water bath through which cold water can be circulated. The hydrogen chloride escaping from the condenser is conducted to a round-bottomed flask containing caustic soda or water. This flask must not be connected to the reflux condenser with an
air-tight seal, or the air cannot escape from the apparatus. The tube from the condenser should discharge directly above the surface of the absorption liquid, but should not dip into it or the liquid will be drawn back into the apparatus.
|
Fig. 5. Laboratory chlorination apparatus. |
The separate parts of the apparatus are connected glass-to-glass, so far as possible, by means of short pieces of thick-walled rubber tubing (pressure tubing). Where longer connections are unavoidable, they should be made with either glass or lead tubing; the latter, befote use, should be cleaned carefully and dried by a blast of air.
A vigorous stream of chlorine is passed through a 500-cc. chlorination flask in which are placed 312 g. (4 moles) dry benzene (the quantity of benzene that can be chlorinated conveniently in the laboratory in the course of a day) and 5 g. iron powder.
After a considerable quantity of chlorine has dissolved in the benzene, the start of the reaction is evidenced by a sudden rise in temperature. The stream of chlorine is diminished and the flow of cooling water is started, after which the introduction of chlorine is again accelerated to such a rate that the reaction temperature is maintained at about 30°C. A much more rapid stream of chlorine leads to incomplete absorption of the gas. Efficient cooling in the reflux condenser is necessary in order to retain as much as possible of the benzene entrained in the hydrogen chloride.
The hydrogen chloride formed in the reaction is led into a 2-liter flask which contains a mixture of 300 cc. sodium hydroxide solution (30° Be) and 700 cc. water, or water alone.
The progress of the reaction is determined from time to time by weighing. For this purpose, the loss in weight of the chlorine cylinder, the gain in weight of the reaction flask, or the gain in weight of the absorption flask, can be determined. In industrial work, the chlorine cylinder is usually weighed, but this may not be possible in the laboratory due to the lack df a suitable balance weighing accurately to 1 gram and having the necessary capacity. Weighing of the absorption flask is usually most suitable, but this procedure can be used only if the chlorine is completely absorbed, as it should be in this experiment. It is recommended that the weight increase of the chlorination flask also be determined as a check at the end of the reaction. It is to be noted that the absorption of one gram atom of chlorine should correspond to a weight increase of the reaction flask of 34.5 grams (Cl minus H), an increase in weight of the absorption flask of 36.5 grams (HC1), and a decrease in weight of the chlorine cylinder of 71 grams (Cl2)-
The reaction is interrupted when 80 per cent of the chlorine calculated as necessary for the formation of monochlorobenzene has been taken up, that is, when the weight of the reaction flask has been increased by 117 grams. If the chlorination is continued beyond this point, the amounts of higher chlorination products increase rapidly and the yield of monochlorobenzene is not increased appreciably. The iron is now allowed to settle and the liquid is decanted into a separatory funnel, washed thoroughly with dilute hydrochloric acid, then with soda solution, and finally with water. The reaction product, weighing about 410 to 420 grams, is subjected to fractional distillation, preferably in vacuum. In order to effect a separation of the mixture in one distillation operation, a column should be used which is at least 60 cm. high, filled with glass rings, and provided with a partial condenser (see page 342). Typically, the following fractions are obtained at atmospheric pressure:
(1) 70-100°, benzene with a little chlorobenzene, about 70 grams
(2) 120-130°, almost pure chlorobenzene, about 295 grams
and at 9 mm. pressure:
(3) up to 55°, mono — and dichlorobenzene, about 10 grams
(4) 55.5°, o — and p-dichlorobenzene, about 40 grams
(5) higher boiling products and residue, very small amount.
By cooling fraction 4 to about 10° and centrifuging, about 12 grams of pure p-dichlorobenzene can be obtained.
The yield of monochlorobenzene is 295 grams, or 65 per cent based on the benzene started with, 84 per cent based on the benzene actually used up in the process.
Technical Observations. Chlorobenzene is an important intermediate in the preparation of a whole series of other compounds (see Section 6 and Tables 1 to 5). It is produced in batches of 2000 kilograms or more in cast iron kettles equipped with agitators and reflux condensers.
Only about 60 per cent of the theoretical amount of chlorine is introduced, whereby the formation of higher chlorination products is greatly reduced and the yield of chlorobenzene, based on the unrecovered benzene, is appreciably increased. The unused benzene is always put back through the process. The rectification is carried out under rigorous control. (Concerning fractional distillation, see also page 341 ff.)
The hydrochloric acid formed in the chlorination is collected in water, and the small amount of chlorine carried over is neutralized by the addition of a small amount of sodium bisulfite. The resulting “chlorination hydrochloric acid” plays an important role in the dye plant. It is cheap and very pure.
The dichlarobenzene which is always farmed in small quantity along with the monochlorobenzene is a mixture of the ortho and para compounds. The latter isomer is easily obtained pure since it is a solid at ordinary temperatures; it is used in the preparation of a series of intermediates (see Section 6 and Table I), and finds use also as a moth repellent. The liquid o-dichlorobenzene, on the other hand, can be freed from the last traces of the para isomer only with great difficulty. It is difficult to manufacture, therefore, and it is generally used only as a high boiling, inert solvent.
An interesting method for chlorination of benzene consists in mixing benzene with chlorine and passing the solution through a contact column filled with iron turnings (Poma-Cesano-Maderno).
The formation of di — and polychlorobenzenes is greatly reduced by mixing the chlorine with a large excess of benzene flowing continuously through a tube, then passing the mixture over the catalyst and into the boiler. The unreacted benzene is continuously distilled off through a good fractionating column and returned through the chlorination chamber, while the chlorobenzene remains in the boiler. Up to 95 per cent of the benzene can be converted to monochlorobenzene in this way.
 15 сентября, 2015
15 сентября, 2015  Pokraskin
Pokraskin 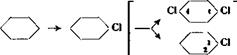
 Опубликовано в рубрике
Опубликовано в рубрике 