Monosubstitution products of benzene exist in only one form, but when a second substituent is introduced into the molecule, any one of three isomers may be formed: ortho, meta and para. Which of the three isomers will be formed in any particular case depends, to a small degree, on the nature of the entering group and on the specific reaction conditions, but it depends chiefly on the nature of the substituent already present. According to this “directing” or “orienting” influence, substituents may be divided into two classes. Groups7 in Class 1 include alkyl, aryl (diphenyl bond, halogen, —OH, —OR, —О—acyl, —NH2, —NHR, —NR2, —NH—acyl, —NR—acyl, —N=N—, and others. These groups direct an incoming substituent exclusively, or nearly so,
to the ortho and para positions. Class 2 includes the groups: —NO-.,, ~S03H, — SO, Cl, — SO, R, —C02H, —C02R, — CONHR, —COR, —CHO, —CN, etc. These groups orient predominately, but seldom exclusively, to the meta position.
Whether the ortho or para position is favored by the Class 1 substituents depends partly on the particular substituent present, but also to a large degree on the nature of the entering group and often on the reaction conditions, especially the temperature. The sulfo group usually enters the para position preferentially, even exclusively in the sulfona — tion of chlorobenzene or toluene or in the sulfonation of phenol at elevated temperatures. On the other hand, sulfonation of phenol in the cold gives an appreciable amount of phenol-o-sulfonic acid. In halo — genation and nitration reactions, both the ortho and para isomers are usually formed; nitration of toluene gives, in addition, a small percentage of m-nitrotoluene.
The directing influence of primary, secondary and tertiary amino groups is greatly weakened by the presence of large amounts of concentrated sulfuric acid. Hence, considerable quantities of the meta isomers are formed in the nitration of amines in concentrated sulfuric acid solution, and in the sulfonation of amines by concentrated sulfuric acid or oleum.
The orienting power of the meta directing substituents is much smaller throughout than that of the Class 1 substituents. This is the reason that, as a rule, ortho and para isomers are formed as byproducts along with the meta compound. ,
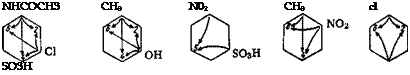 |
If there are already two substituents in the benzene ring, they may either direct a third entering group to the same position, or work in opposition to each other. The former is the case if the two groups already present belong to the same class and are located meta to each other, or if one group belongs to Class 1 and the other to Class 2 and they are located ortho or para to each other, for example:
In these cases, the new substituent is directed to the position favored by the joint action of the two groups already present, as indicated by the arrows in the formulas.
![]()
![]()
![]()
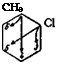
If, however, the two groups already present belong to the same class and are ortho or para to each other, or if they belong to different classes and are meta to each other, as in the following examples:
then they will exert opposing influences. Usually this results in the formation of all possible isomers. If one of the substituents is a hydroxyl group, however, its influence outweighs that of the other substituents and is the determining factor. Thus, with p-cresol, p-chlorophenol, and p-acetaminophenol, any new substituent enters exclusively the position ortho to the hydroxyl. The directing power of the hydroxyl group is distinctly reduced by alkylation or acylation. Such substituted hydroxyl groups have about the same directing influence as free or acyl — ated amino groups. Halogen and alkyl groups are very similar in their directing activity; if they are located in a molecule so that they have opposing directing effects, a mixture of the possible isomers in nearly equal amounts is always obtained (e. g., in the nitration of p-chloro — toluene).
As mentioned before, the very strong directing effect of the amino group is significantly reduced by the presence of large quantities of concentrated sulfuric acid. This is true especially for free amines, but also to a smaller extent for their acyl derivatives. Thus, aceto-p- toluidide is nitrated by aqueous nitric acid exclusively in the position ortho to the acetamino group; in concentrated sulfuric acid solution, however, a considerable quantity of 2-nitro-4-acetaminotoluene is formed along with the 3-nitro compound. p-Toluidine itself gives
2- nitro-4-aminotoluene almost exclusively when it is nitrated in the presence of much concentrated sulfuric acid (see page 165). The behavior of p-chloroaniline and its acetyl derivatives is similar. In the sulfonation of amines with concentrated sulfuric acid or oleum, the influence of the other substituents may become important or even predominant. On the other hand, in sulfonation by the baking process (see page 126), the sulfo group always enters a position ortho or para to the amino group, regardless of other substituents. Also, the formation of m-nitroaniline derivatives can be avoided completely if the arylsulfonyl (especially the p-toluenesulfonyl) derivative of the amine
is nitrated with nitric acid in water or an organic solvent.8 (p-Toluene — sulfanilides behave like phenols in many respects; they dissolve in caustic alkalis, couple with diazonium compounds, etc.)
Of the different isomers which might be formed, according to the above rules, by further substitution into disubstituted derivatives, the unsymmetrical (1,2,4) derivatives are strongly favored. Symmetrical (1,3,5) trisubstitution products are formed only with difficulty, and vicinal (1,2,3) derivatives are generally formed only as by-products in insignificant amounts. (The chlorination of o-nitrotoluene is an exception, see page 160.)
When substituents of the two classes are working in opposition, the influence of the Class 1 groups usually predominates, or completely masks the influence of the Class 2 groups. ,
The same principles which govern the introduction of a third group into disubstituted benzene derivatives hold, in general, for the further introduction of a fourth group into trisubstituted derivatives. It is to be observed that here there is a strong tendency for the formation of symmetrical (1,2,4,5) derivatives. These are often the chief products even in cases where predominant formation of other isomers might be expected on the basis of the directing influence of the three groups already presen^. Thus, nitration of 2-chlorotoluene-4-sulfonic acid gives chiefly 2-chloro-5-nitrotoluene-4-sulfonic acid, even though the methyl and sulfo groups both direct to the 6 position, and only the chbro directs to the 5 position. The same results are obtained in the n, tra — tion of l,2-dichlorobenzene-4-sulfonic acid, 2-chloro-4-nitrotoluene, etc.
 13 сентября, 2015
13 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 