When a previously unknown compound has been made for the first time, the only criterion of its purity and homogeneity is the constancy of its properties after repeated purification operations. The compound is subjected to the various purification procedures (distillation, recrystallization from as many different solvents as possible, conversion into salts, esters, amides, etc., and regeneration), and is regarded as pure and homogenous if its properties remain unchanged after all these treatments. Even then one cannot be absolutely certain. Again and again, compounds, which have been assumed to be pure, are proven to be mixtures by later research using more refined methods.
In the industrial laboratory, however, the work more often involves compounds which are described in the literature. In such cases it suffices, as a rule, to establish an agreement between the properties of the compound prepared and those described in the literature. The physical constants are the properties which are most easily expressed numerically, and of these, the melting point and the boiling point are the most easily determined.
The determination of the boiling point has already been mentioned briefly in the discussion on distillation, and here we shall deal with the corrections which may be necessary. It is well known that the boiling point is dependent on the pressure and therefore the pressure must be taken into account in an accurate boiling point determination. This correction is not very large, not exceeding about 2°C., at barometric pressures above 700 mm. A larger error under some conditions
may be caused by the fact that only the bulb, and not the whole thermometer, is immersed irt the vapor of the boiling substance. A correction must be made for the part of the mercury column, the so-called “exposed stem,” which is not heated to the temperature of the vapor. The correction is proportional to the length (f) of the exposed stem (in degrees) and to the difference between the boiling point and room temperature (£). The correction is calculated by the formula:
correction = 0.00015ft
It is seen that the correction may be quite large when the boiling point is high. Whenever a boiling point is given, the pressure at which it was taken should be stated, and an indication given as to whether or not a correction for the exposed stem has been applied.
There are instances where a correct boiling point does not demonstrate the purity of a substance. This might apply to cases involving mixtures of substances which boil at the same, or nearly the same temperature, so that the presence of a mixture is not detected from the boiling point. However, if one has prepared a substance, one knows, as a rule, whether such a mixture can be present; and if there is this possibility, the boiling point is not used as a criterion for purity. If, on the other hand, the presence of a by-product having the same boiling point is excluded, then a correct, sharp boiling point may be taken as evidence that no appreciable quantities of impurities are present, and that the material is sufficiently pure for technical use. To be sure, complete purity is not demonstrated, since small amounts of a much higher boiling impurity might go over with the vapor of a lower boiling compound and this would not be apparent from the boiling point. If the specifications call for very high purity, the determination of boiling point is not adequate.
The melting point is much more sensitive to impurities than the boiling point. Just as the freezing point of a liquid is lowered by dissolving a foreign substance in it, so is the melting point of a solid material lowered by the presence of an impurity which is soluble in die melt. A melting point which is too low, therefore, is infallible evidence for the presence of an impurity. Some impurities which are not dissolved in the melt have no effect on the melting point. There are other cases where two isomeric, homologous, or otherwise closely related substances are isomorphous and form mixed crystals, or form a molecular compound which has a characteristic melting point and behaves in every respect like an individual compound. A particularly striking ex-
ample of this phenomenon is afforded by acetyl-m-aminophenol and n- butyryl-m-aminophenol. These two compounds melt at 145° and 138°C., respectively, and an equimolecular mixture of the two melts at 154- 155°C. Molecular compounds of this type cannot, as a rule, be separated into their constituents by recrystallization, and their non-homogeneity is usually detected only by conversion to derivatives. Such cases do not occur often, but they are not so rare as is generally assumed; the possibility should always be kept in mind if errors are to be precluded.
Another possibility for difficulty in melting point determinations lies in the presence of solvent of crystallization. It is well known that many compounds crystallize with water of crystallization, or with alcohol or benzene of crystallization, etc. Frequently, the solvent of crystallization is driven off below the melting point and does not affect the latter. In other cases, however, an apparent melting, or solution in the solvent of crystallization, occurs far below the true melting point. A well known example of this behavior from inorganic chemistry is afforded by soda crystals which melt very easily in their water of crystallization. In organic chemistry, it is mainly the high molecular compounds, such as those of the triphenylmethane type, which stubbornly retain solvents of crystallization. A compound of this nature melts at different temperatures depending on the particular solvent of crystallization present, and hence on the solvent from which it was recrystallized. Such a situation can give rise to great confusion if the circumstances are not considered, and therefore melting points should be determined only with samples which have been dried to remove all of the solvent of crystallization.
Finally, difficulties are encountered in determining the melting points of substances which begin to decompose below their melting points. An example of such a compound is phthalic acid which melts at 206-208°C., but which begins to split out water far below this temperature with the formation of its anhydride which melts at 131°. If the melting point apparatus is heated slowly and carefully, and the test sample remains in the warm bath for a long time, the compound is completely converted to the anhydride long before the true melting point of the acid is reached, and the sample gives the appearance of having melted. The melting point obtained is therefore much too low. In such cases, the bath should be preheated to a temperature only slightly below the melting point of phthalic acid before introducing the test sample. Then the heating should be continued as rapidly as possible, so that the acid has little time to undergo anhydride formation, but melts first. Only in this way can one obtain the correct melting point value.
In all the numerous cases where melting with decomposition occurs (usually with high melting compounds), the possibility always exists that what is observed is not the true melting point of the material, but the point at which the compound is decomposed into liquid products. Decomposition points do not depend solely on the temperature, as do true melting points, but also on the length of heating, and the more rapidly the heating is carried out, the higher will be the observed “melting point.”
The difficulties which have been mentioned are only partly to blame for the fact that the literature often gives several melting points for a compound, differing from each other by several degrees. A much greater part of the trouble arises from the fact that substances, not entirely pure, were regarded as pure originally. In addition, an appreciable part of the disagreement can be ascribed to the different methods which have been used for determining melting points. First, it must be remembered that the correction for the mercury column not immersed in the heating bath is just as important here as it is in boiling point determinations, but this fact has not always been borne in mind. The necessity for this correction can be eliminated to a certain extent by the use of the shortened thermometers introduced by Zincke. These thermometers cover only a narrow temperature range, for example,
0- 50°, 50-100°, 100-150°C., etc., so that the exposed stem is never more than about 50° long. Under these conditions the correction is so small that it can be disregarded. Of course, one must have a complete set of these thermometers, and must know beforehand approximately what the melting point is, in order to be able to select the correct thermometer. The chief use for the Zincke thermometers is in the final checking of a melting point, perhaps before publishing it.
A further source of error in melting point determinations is introduced if the temperature in the melting point tube and in the thermometer bulb is not the same. This difficulty is avoided most easily by using as a heating bath an open beaker with a stirrer so that the entire bath is heated uniformly. The apparatus must be kept open so that the stirrer can be installed without difficulty, and therefore a bath fluid must be used which is neither volatile nor hygroscopic. Practically, one is limited to paraffin oil which is quite satisfactory for moderate temperatures. It rapidly turns dark at higher temperatures, however, so that it must be renewed frequently when melting points around 200°C. are being determined. An enclosed apparatus is more suitable under these conditions because it permits the use of concentrated sulfuric acid as the bath fluid. Sulfuric acid remains colorless even when heated
 |
 |
to its boiling point (about 280°) and, if it should become colored due to contamination by organic materials, it can be decolorized by adding a small particle of saltpeter. The simplest closed apparatus consists of a small round-bottomed flask with a long narrow neck in which the thermometer is held by a cork (Fig. 2). The cork should have a narrow groove cut in it so that pressure is not built up inside the flask. Satisfactory results can be obtained with the apparatus if care is taken to apply the heat slowly and uniformly, by using either a small flame directly under the flask or a larger flame kept in constant motion. It is
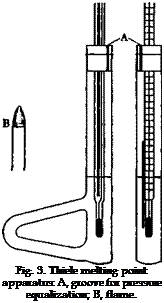
also necessary that the melting point tube be placed so that the sample is at the same height as the middle of the mercury bulb. It has been found that uniform heating of a bath can be obtained by applying the heat, not directly under the thermometer, but to an attached side piece, whereby the bath fluid is made to circulate. The Thiele apparatus is built to make use of this principle (Fig. 3). More accurate tests show, however, that the circulation of the liquid is not entirely uniform, and frequently the side of the tube to which the side piece is attached becomes appreciably hotter than the opposite side. One must be sure, therefore, that the melting point tube is always located in the same place, preferably in the exact center of the tube. If two samples are to
be compared, both of them should be placed at the same distance from the openings into the side piece.
In recent times, apparatus which make use of a metal block, usually copper, instead of a liquid bath medium, have come to be preferred (Fig. 4). The melting point tube is placed in a narrow slit through
t
!
D
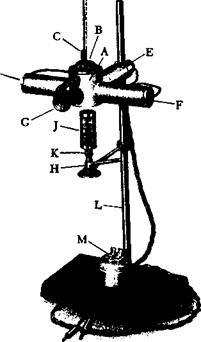 Fig. 4. Iseli melting point apparatus, with metal block and three-point illumination. Only the side lights are used until sintering begins, and then the rear light is turned on to permit close observation of the formation of a clear melt. The apparatus is also built with an electrical heating element.
Fig. 4. Iseli melting point apparatus, with metal block and three-point illumination. Only the side lights are used until sintering begins, and then the rear light is turned on to permit close observation of the formation of a clear melt. The apparatus is also built with an electrical heating element.
A, block ^
B, block closure
C, 3 melting point tubes
D, thermometer
E, rear light
F, side lights C, magnifier H, microbumer
J, mica cylinder
K, air control sleeve
L, stand
M, switch for lights.
which the melting can be observed, and the thermometer is inserted in a hole directly behind the slit. An apparatus such as this has the great advantage that it can be used for temperatures above the boiling point of sulfuric acid, 280°. Care must be exercised in using it, however; heat must be applied very slowly and uniformly. This may be accomplished by electrical heating, using a constant current, or a very small gas flame
(microbumer), always placed in the same position. If the thermometer is then calibrated by melting, under constant conditions, a series of absolutely pure compounds having accurately known melting points, the melting point block is capable of giving reliable results.
Whichever apparatus is used, the safest procedure is always to compare the melting point of the product being tested with that of an authentically pure sample of the same material. The two samples should be melted simultaneously in the same apparatus and under exactly Ле same conditions. Melting point tubes are selected which have as nearly as possible the same diameter and are filled to equal height, taking care that both samples are equally packed. The packing may be done by holding the tubes between two fingers and tapping lightly on the table, or by dropping them onto the table through a fairly long glass tube. The two tubes are attached to the thermometer at the same height, and at the same distance from the heat source.
The same precautions should be observed in establishing the identity of two substances by the so-called mixed melting point. In this case, melting point tubes are filled with the two substances, and a third tube with a mixture of the two substances. The melting points of all three samples are determined simultaneously in the same apparatus. If the mixture melts at a lower temperature than the two individual substances, then the two substances are not identical. If the mixture has the same melting point as the individual substances, it is highly probable that the two materials are identical. This conclusion cannot be drawn with absolute certainty, however, because it could be that the two materials are different but are not mutually soluble in the melt, or that they form mixed crystals or a molecular compound accidentally having the same melting point.® One should not be satisfied, therefore, with a mixed melting point determination alone, but should evaluate other properties as well for better control.
The beginner is often uncertain as to which phase of the melting should be taken as the melting point The following definition might be given. Melting begins when the substance on the wall of the tube begins to flow down; melting is ended when a completely clear melt can be observed. The temperature interval between the beginning and end of the melting is stated as the melting point. As soon as melting begins, the heating should be interrupted and the temperature maintained as nearly constant as possible. The heating is then carefully continued only when it is seen that the melting is not going to be complete. Pure, homogeneous substances, which melt without decomposition,
5 Cf. Kofler and Brandstatter, Ber., 75, 496 (1942).
have sharp melting points as a rule; the interval between beginning and end of melting is about 0.5° at the most. This temperature range may be much larger with impure materials or with compounds which melt with decomposition. Frequently, a substance softens and shrinks before melting; this should be designated as sintering, not as melting. An accurate statement of the melting point would read, for example: m. p., 124-125°, sinters at 118°.
The infrequently used microdetermination of melting point by the method of Kofler6 can only be mentioned here.
Insofar as it is applicable and despite all of its attendant uncertainties the determination of melting point is the most widely used control method because of the ease and rapidity with which it can be carried out. It cannot be used, of course, with substances which are liquid at ordinary temperatures or with solid substances which carbonize or otherwise decompose before melting. Most of the sulfonic acids and their metal salts belong to the latter group. Many liquid substances are distillable without decomposition and with these the boiling point may be used as a criterion of purity. In-addition, the determination of density, which is easy to perform, is valuable for purity control if a large enough sample is available. The refractive index and, in the case of optically active materials, the optical rotation, are more difficult to determine, but are very sensitive to the presence of impurities. These latter two properties are of great value for identification and purity control in the terpene field, for example.
Other optical properties to be considered, for both solids and liquids, are color and fluorescence. The great majority of organic compounds are colorless when in a pure state, but crude products are always more or less colored. Most of the colored impurities are removed by the ordinary purification operations (distillation, reprecipitation, recrystallization, perhaps using animal charcoal, etc.), but frequently a slight coloration remains and is not removable without a disproportionately large loss of product. Now a very weak coloration in a substance, which is colorless when pure, is an indication of lack of complete purity. The quantity of colored impurity, however, may be so small that it causes no trouble even for pure research purposes and is completely unimportant in technical work. Naturally, it is difficult to say how strong a coloration is permissible. It might be said that a substance is suitable for technical purposes if its solutions do not appear appreciably colored when viewed in thin layers or single drops. In many in — [3] stances, a stronger coloration does no harm; the decision depends on the particular application and must be made for each individual case.
The determination of absorption spectrum is very valuable for the identification of products which are colored in themselves, such as the the true dyes. It is of less value as a means of controlling purity because the position of the absorption bands is frequently not shifted by the presence of impurities. Test dyeing is used industrially as the chief method for testing dyes for purity, provided that a sample of the pure dye is available for comparison.
Fluorescence is often a valuable indicator of purity, and can often be used for differentiating and recognizing isomeric or closely related compounds, especially those in the naphthalene series. For example, the alkaline solutions of /J-naphthol and almost all of its sulfonic acids show strong fluorescence; only those sulfonic acids having the sulfo group in the 1 position show no fluorescence. Hence, in the preparation of 2-naphthol-l-sulfonic acid from /З-naphthol, the fact that the product has no fluorescence shows that it contains neither unchanged /3- naphthol nor an isomeric sulfonic acid (see page 199). Furthermore, sodium 2-naphthol-6-sulfonate (Schaeffer salt) in aqueous solution exhibits only a very weak violet-blue fluorescence. The salts of both disul — fonic acids formed simultaneously (2-naphthol-3,6-disulfonic acid, R salt, and 2-naphthol-6,8-disulfonic acid, G salt) fluoresce much more strongly and with a greenish blue color. The fluorescence of these compounds is increased by the addition of soda, but the character of the fluorescence is unchanged. The presence of a very small amount of R salt or G salt in Schaeffer salt is enough to mask the violet-blue fluorescence completely. Thus, a recognizable violet-blue fluorescence with Schaeffer salt shows immediately that it is practically free from disul — fonic acids (cf. page 196).
With all crystalline substances, the crystal form is an intrinsic indication of purity. Impurities are often recognized macroscopically, but more generally their presence is revealed by microscopic observation of a different crystal habit or amorphous character. There are cases where the impurity crystallizes with the main product and is not directly detectable. Such mixed crystals, however, frequently have a different crystal form from that of the pure compound, and are distinguishable if a direct comparison is made with a pure sample. The control of purity of sulfonic acids, which is made difficult by the lack of characteristic melting points, is simplified somewhat by the fact that numerous salts can be prepared from them, and one or more of these will usually be found to have a recognizable characteristic crystal form.
All tests for purity are made easier if a comparison sample of authentic purity is available. If such a sample is not available, it can be prepared by careful purification of a portion of the product itself. One bothersome, but generally applicable, method of testing for purity is carried out by recrystallizing a sample of the product being tested, and comparing the recrystallized material with that retained in the mother liquor. To this end, the mother liquor may be fractionally evaporated, whereupon the impurities, as a rule, will become so concentrated in the last mother liquor that they are easily detected.
Of course, the control of purity must be based not only on the physical properties discussed above, but also on the chemical behavior of the substance being tested. It is extremely difficult to generalize on this point, however. The testing methods must be adapted to the chemical properties of the particular compound being tested and of the impurities suspected to be present; all this requires a general knowledge of the reactions of organic chemistry, which cannot be supplied here. Mention might be made of only one reaction, namely, the formation of azo dyes. This reaction is not frequently encountered in scientific literature but is often used in industrial laboratories with good results. The reaction can be applied to all compounds which are capable of being diazotized or capable of coupling with diazonium compounds. In many cases, impurities likely to be present, such as isomers, give dyes which are sufficiently different from the dyes yielded by the main product to be distinguishable. The components suitable for each individual case must be established by test experiments, and this requires some practice. Schaeffer salt may again be used as an example. We have already seen how an admixture of R salt in Schaeffer salt is recognized. How can the reverse situation be detected, i. e., the presence of Schaeffer salt in R salt? Completely pure R salt reacts in carbonate solution with the diazonium compound from monoacetyl-p-phenylenediamine to give a bluish red dye which is practically insoluble in the reaction mixture which contains some salt. When the dye is filtered off, a pale rose colored filtrate is obtained. Schaeffer salt, treated in the same way, yields a more soluble, yellowish red dye which is not fully precipitated under the conditions of the experiment. If the R salt being tested contains even a little Schaeffer salt, then one obtains, after filtering off the dye, a yellowish colored filtrate much stronger in color that when pure R salt is used. The amount of Schaeffer salt present in the original sample can be estimated by the depth of color in the filtrate.
The beginner in the industrial laboratory frequently wants to know when a product which is not completely pure may be designated as
sufficiently pure for technical purposes. A product is sufficiently pure if it is usable for the particular purpose at hand, and hence, if the impurities present do not interfere in the proposed use of the compound. Thus, a great deal depends on the intended application of the compound. The same product might be sufficiently pure for one use, and entirely unsuitable for another. Furthermore, a product considered quite satisfactory for a certain purpose might be unusable if the procedure is changed. The technical applicability varies from case to case, and can be determined only by suitable experiments.
As a starting point for the beginner, it might be said that substances which melt 2 to 3° too low are, in general, suitable for industrial use. It has already been mentioned that moderate color in the product does no damage. Finally, it may be pointed out that inorganic salts, always present in products isolated by salting out, have no damaging effect in most applications, and are generally not even regarded as impurities. Such salts need be considered only with respect to their effect on the quantity of starting material to be used.
 8 сентября, 2015
8 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 