Azo pigments, both numerically and in terms of tonnage produced, dominate the yellow, orange and red shade areas in the range of commercial organic pigments (Chapter 3). The chemical structures of some important classical azo pigments are shown in Figure 9.1. The structures are illustrated in the ketohydrazone form since structural studies carried out on a wide range of azo pigments have, in each case, demonstrated that the pigments in the solid state exist exclusively in this form. Many other colour chemistry texts follow the commonly used convention to illustrate them in the azo tautomeric form. Simple classical monoazo pigments such as Hansa Yellow G, 206 (C. I. Pigment Yellow 1) and Toluidine Red, 207 ( C. I. Pigment Red 3) are products which show bright colours and good lightfastness, but rather poor solvent resistance. The good lightfastness of these molecules is attributed to the extensive intramolecular hydrogen-bonding in the form of six-membered rings. The inferior fast-
|
Figure 9.1 Chemical structures of some important classical azo pigments |
ness to organic solvents is due to their small molecular size and the fact that the intermolecular interactions in the crystal structures involve essentially only van der Waals’ forces. The use of these pigments is largely restricted to decorative paints. The pigments resist dissolving in the solvents used in these paints (either water or aliphatic hydrocarbons) at the low temperatures involved, but have a tendency to dissolve in more powerful solvents, especially if higher temperatures are involved.
The most important yellow azo pigments, particularly for printing inks but also for a range of paint and plastics applications are the dis — azoacetoacetanilides (Diarylide Yellows), which include C. I. Pigments Yellow 12, 208a, and 13, 208b. These pigments have been shown to exist in bisketohydrazone forms, structurally analogous to Hansa Yellow G (206). They exhibit higher colour strength and transparency than the corresponding monoazo pigments, and improved solvent fastness which is attributable to the larger molecular size. The Pyrazolone Oranges, such as C. I. Pigment Orange 34 (209), are similar both in chemical structure and in properties to the Diarylide Yellows and are used in a similar range of applications. The Naphthol Reds, of which C. I. Pigment Red 170 (210), is an important example, are structurally related to Toluidine Red (207). Compound 210 shows superior solvent resistance as a result of its larger molecular size and due to the presence of the amide groups which provide strong intermolecular forces of attraction in the crystal lattice structure. Consequently, it is suitable for use in a wider range of paint and plastics applications. The most important of the classical red azo pigments are metal salts, such as compounds 211a-c, which are derived from azo dyes containing — SO3~Na+ or — CO2~Na + groups by replacement of the Na + ions with divalent metal ions, notably Ca2+,Sr2+,Ba2 + and Mn2+. They are products of high colour strength and brightness, high transparency and good solvent resistance. They are especially important for printing ink applications, C. I. Pigment Red 57:1 (211a), for example, being the main pigment used to provide the magenta inks for multicolour printing. Metal salt pigments have evolved from ‘lake’ pigments, now largely obsolete, which were essentially anionic azo dyestuffs precipitated onto inorganic substrates such as alumina and barium sulfate.
 16 декабря, 2015
16 декабря, 2015  Pokraskin
Pokraskin 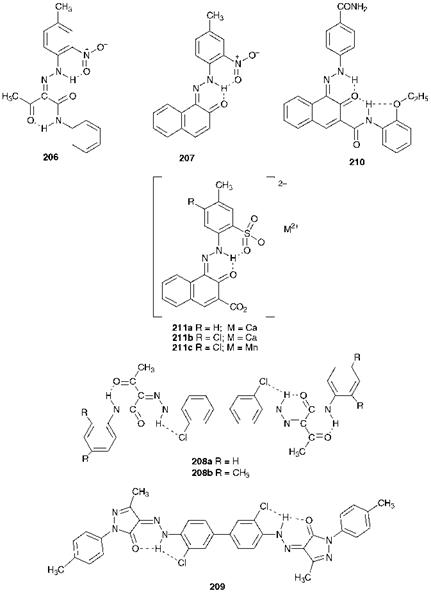
 Опубликовано в рубрике
Опубликовано в рубрике 