A characteristic feature of the chemistry of aromatic ring systems is their tendency to undergo substitution reactions in which the aromatic character of the ring is retained. Since most aromatic systems are electron-rich in nature, by virtue of the system of re-electrons, the most frequently encountered reactions of aromatic compounds are electrophilic substitution reactions. Nucleophilic aromatic substitution reactions are less commonly encountered. For reaction with nucleophiles under mild conditions, aromatic systems require the presence of features which reduce the electron density of the re-system to facilitate nucleophilic attack. For example, chlorobenzene undergoes nucleophilic substitution (e. g. of Cl by OH) only under vigorous conditions. In contrast, 1-chloro-2,4-dinitro — benzene undergoes nucleophilic substitution very readily because of the activating effect of the two powerfully electron-withdrawing nitro groups. Reactions of this type are used in the synthesis of some nit — rodiphenylamine dyes (see Scheme 6.5 in Chapter 6). Another feature of nucleophilic substitution reactions illustrated by this particular reaction is the requirement for a good leaving group, such as the chloride anion.
By far the most important fibre-reactive groups which react by nucleophilic substitution contain six-membered aromatic nitrogen-containing heterocyclic rings with halogen substituents. The first group of commercial reactive dyes, the Procion dyes, which remain among the most important in use today, contain the 1,3-5-triazine (or s-triazine) ring, a six-membered ring of alternate carbon and nitrogen atoms, containing at least one chlorine substituent, and with the amino (-NH-) group as the bridging group. The reaction of a chlorotriazinyl dye 173 with the cellu — losate anion, incorporating an outline mechanism for the reaction which is characteristic of aromatic nucleophilic substitution, is illustrated in Scheme 8.1. A number of features may be identified as responsible for facilitating the nucleophilic substitution reaction. Firstly, the electron — withdrawing nature of the heterocyclic nitrogen atoms and, to a lesser extent, of the chlorine atom reduces the electron density in the aromatic ring which is thereby activated towards nucleophilic attack. An important feature is the extensive resonance stabilisation of the anionic intermediate 174. In particular, it is of special importance that the delocalised negative charge is favourably accommodated on the electronegative heterocyclic nitrogen atoms in each of the three contributing resonance forms. In the final step of the sequence, the chloride ion, a particularly good leaving group, is eliminated. As a result of the reaction, a dye-fibre covalent (C-O) bond is formed, as illustrated for structure 175a, and it is to this that the excellent fastness to wet-treatments may be attributed.
Both dichloro — and monochlorotriazinyl dyes are used commercially. Dichlorotriazinyl dyes (marketed as Procion M dyes), 173a, are more reactive than the monochlorotriazinyl (Procion H) types 173b. This may be explained by the fact that, in the monochloro dyes 173b, the group X is electron-releasing, commonly amino (primary or secondary) or methoxy, replacing the electron-withdrawing chloro group in the dichloro derivatives (173a). There is therefore an increase in the electron density of the triazine ring in the monochlorotriazinyl dyes, which reduces their reactivity towards nucleophilic attack. Procion M dyes are capable therefore of reaction with cellulosic fibres at lower temperatures than is required for the Procion H dyes. Procion M dyes are thus applied at temperatures of around 40-60 °C whereas Procion H dyes are applied at around 80-90°C.
A feature of the chemistry of triazinyl reactive dyes, which is in fact common to all reactive dye systems, is that they undergo, to a certain extent, a hydrolysis reaction that involves reaction of the dye with OH _ anions present in the aqueous alkaline dyebath in competition with the dye-fibre reaction. The hydrolysis reaction is also illustrated in Scheme
8.1. Reactive dye hydrolysis is a highly undesirable feature of reactive dyeing for a variety of reasons. In the first instance, the hydrolysed dye 175b which is formed is no longer capable of reacting with the fibre and so must be washed out of the fibre after dyeing is complete, to ensure the
|
|
excellent fastness to washing. A second reason is that a degree of fixation less than 100% and the need for the wash-off after treatment reduces considerably the cost-effectiveness of the process. Finally, the hydrolysed dye and any unreacted dye inevitably ends up in the dyehouse effluent, and this presents environmental concerns to which society is becoming increasingly sensitive (Chapter 11).
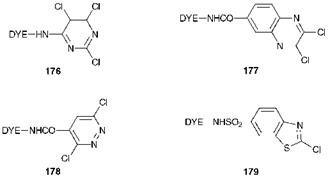
The initial success of the reactive dyes based on the triazine ring system was immediately followed by intense research activity into the possibilities offered by other related nitrogen-containing heterocyclic systems. Numerous systems have been patented as fibre-reactive groups although only a few of these have enjoyed significant commercial success. Some examples are illustrated in Figure 8.2. They include the trichloro — pyrimidines 176, the dichloropyridazines 177, the dichloroquinoxalines 178 and the chlorobenzothiazolyl dyes 179.
Perhaps the best known of these related groups of reactive dyes are those which utilise the 2,4,5-trichloropyrimidinyl (2,4,5-trichloro-1,3-dia — zinyl) system, 176, the basis of the Drimarene dyes marketed originally in
|
Figure 8.2 Structures of some other heterocyclic reactive dye systems |
|
Figure 8.3 Structure of the intermediates formed from the reaction of trichloropyrimidinyl dyes with nucleophiles |
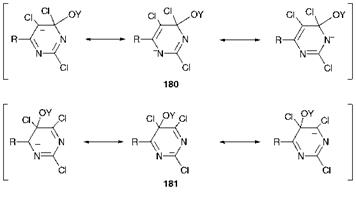
the 1960s by Sandoz. These dyes react with cellulosate anions by nucleophilic displacement of either the 2- or 4-chlorine atoms according to a mechanism analogous to that shown in Scheme 8.1 for triazine dyes. In contrast, the chlorine atom at the 5-position is not readily substituted. The reasons for this are immediately apparent from examination of the resonance forms of the intermediates derived formally from attack of the nucleophile at the relevant carbon atom as illustrated in Figure 8.3. There are three contributing resonance forms in each case. In the anionic intermediate 180, which is formed by attack at the 4-position, two of the three contributing resonance forms have the negative charge accommodated favourably on the electronegative nitrogen atoms. The reader might like, as an exercise, to demonstrate that a similar situation arises from attack at the 2-position. In contrast, however, attack at the 5- position gives an intermediate 181 in which the negative charge is local-
ised on carbon atoms in each of the three contributing forms. The instability of this intermediate explains the lack of reactivity at the
5- position. The 5-chloro substituent, although unreactive, is nevertheless of technological importance. Since the fibre reactive group has only two activating heterocyclic nitrogen atoms (compared with three in the Procion dyes), the 5-chloro substituent serves to enhance the reactivity of the ring towards nucleophilic attack as a result of its electron-withdrawing nature and ensures that the dyes are capable of reacting with cellulose at reasonable temperatures. It is of interest that in the cases of reactive dye systems 177-179 the bridging group is electron-withdrawing (carbonyl or sulfonyl) and no doubt plays a decisive part in activating the system to nucleophilic attack, ensuring that dyes have appropriate reactivity for the fibre.
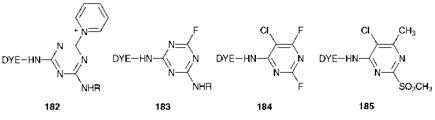
Another method whereby the reactivity of reactive dyes may be modified involves the use of alternative leaving groups other than the chloride ion. Some examples of reactive dyes using this modification are illustrated in Figure 8.4. One of the earliest ventures in this field involved the use of quaternary amino groups, as for example in the triazine derivative 182. Dyes using systems of this type were found to have enhanced reactivity towards cellulosic fibres, but their commercial development was inhibited by a degree of nervousness concerning the liberation of tertiary amines, pyridine in this case, into the dyebath. Some commercial success has been achieved using fluorinated heterocycles, such as those found in the triazine 183 (Cibacron F dyes, Ciba) and the pyrimidine 184 (Levafix EA dyes, Bayer) in spite of the severe hazards which needed to be overcome in their manufacture. Because of the high electronegativity of the fluorine atoms, these dyes show particularly high reactivity and are therefore capable of fixation to cellulosic fibres under mild conditions, for example at relatively low temperatures. Another leaving group which has been utilised to produce highly activated dyes is the methylsulfonyl group (-SO2CH3), used in Levafix P dyes (Bayer) which are based on the pyrimidine structure 185.
|
|
Fibre-reactive Groups Reacting by Nucleophilic Addition
Alkenes are generally regarded as relatively reactive compounds, their reactivity being attributable to the presence of the C=C double bond. Characteristically, alkenes readily undergo addition reactions, most commonly of the electrophilic type because of the electron-rich nature of the re-bond. Nucleophilic addition reactions of alkenes are less commonly encountered but can take place when there are strongly electron-withdrawing groups attached to the double bond, thereby reducing the electron density and thus facilitating nucleophilic attack. Nucleophilic addition to substituted alkenes of this type is alternatively referred to as either conjugate addition or Michael addition.
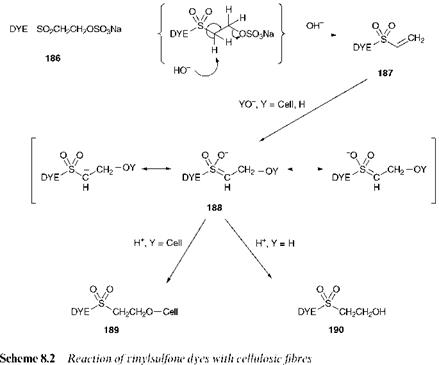
The most important reactive dyes in commercial use for application to cellulosic fibres in which the fibre-reactive groups react by nucleophilic addition are the Remazol reactive dyes. These dyes, based on the vinylsul — fone reactive group, were introduced by Hoechst soon after the launch of the Procion dyes based on the triazine system by ICI. The chemistry of the process in which vinylsulfone dyes react with cellulose under alkaline conditions is illustrated in Scheme 8.2. The dye is supplied by the manufacturers as the ^-sulfatoethylsulfone 186, which is not itself fibre-reactive and is commonly referred to as the stable storage form. The water — solubilising sulfonate group, as a sulfate ester, is in the case of these dyes attached to the latent fibre-reactive group. As illustrated mechanistically in the scheme, the reaction of compound 186 with aqueous alkali causes its conversion by an elimination reaction into the highly reactive vinylsul — fone form 187. The presence of the powerfully electron-withdrawing sulfone group activates the double bond towards nucleophilic attack. Attack by the cellulosate anion on the vinylsulfone initially leads to the anionic intermediate 188 which is stabilised by resonance, with important contributions from canonical forms in which the negative charge is delocalised on to the electronegative oxygen atoms of the sulfone group. The addition reaction is completed by protonation, and the ultimate outcome is the permanent formation of a covalent dye=fibre (C=O) bond as illustrated by structure 189. In this reactive dyeing process, as with dyes which react by nucleophilic substitution, hydrolysis also takes place in competition with the dye=fibre reaction. This reaction, leading to hydrolysed dye 190, is also illustrated in Scheme 8.2.
There are relatively few other reactive groups which react by nucleophilic addition and which have achieved significant commercial success. However, one particular system which is worthy of note is the a-bromoacrylamido group, found in the Lanasol dyes (Ciba), which are among the most widely used reactive dyes for wool. The chemistry
|
|
involved in the reaction of these dyes with the amino groups present on the wool fibre is outlined in Scheme 8.3. As well as the nucleophilic addition reaction, there is the possibility that the second bromine atom may be replaced by nucleophilic substitution leading, in principle, to cross-linking of the fibre, so that the dyes may be considered as bifunctional.
 11 ноября, 2015
11 ноября, 2015  Pokraskin
Pokraskin 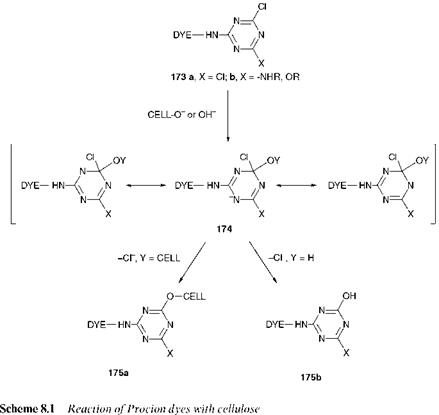
 Опубликовано в рубрике
Опубликовано в рубрике 