It is probable that history will judge the development of reactive dyes to have been the most significant innovation in textile dyeing technology of the 20th century. As a consequence of their particular importance, and because they make use of some interesting organic chemistry, this chapter is devoted entirely to a consideration of the chemical principles involved in the application of reactive dyes. Reactive dyes, after application to the fibre, are induced to react chemically to form a covalent bond between the dye and the fibre. This covalent bond is formed between a carbon atom of the dye molecule and an oxygen, nitrogen or sulfur atom of a hydroxy, amino or thiol group on the polymer. Because of the strength of the covalent bond, reactive dyes once applied to the textile material resist removal and as a consequence show outstanding washfastness properties. Initially, reactive dyes were introduced commercially for application to cellulosic fibres, and this remains by far their most important use, although dyes of specific types have also been developed for application to protein and polyamide fibres. The potential to apply reactive dyes to other fibre types, including polyester and polypropylene, has been demonstrated technically although they are not as yet a commercial reality.
The concept of attempting to link dye molecules covalently to fibre molecules to produce colours with superior fastness to washing had been envisaged long before the 1950s when the major breakthrough took place. Prior to this, however, attempts at dye fixation employed rather vigorous conditions under which serious fibre degradation occurred. Success was first achieved in 1954, when Rattee and Stephen demonstrated that dyes containing the 1,3,5-triazinyl group were capable of reaction with cellulosic fibres under mildly alkaline conditions and under these conditions no significant degradation of the fibre was observed. The launch by ICI of Procion dyes based on this reactive system proved to be an almost immediate success. The new dyes were capable of providing
superior washfastness compared with direct dyes, a much wider range of brilliant colours than were available from vat and azoic dyes, and they were capable of both continuous and batchwise application. This success provided the impetus for a considerable body of research by most dye manufacturers into the development of alternative types of fibre-reactive groups.
A general schematic representation of the structure of a reactive dye is illustrated in Figure 8.1. In the figure, four important structural features of the molecules may be identified separately: the chromogen, the water — solubilising group, the bridging group and the fibre-reactive group. The chromogen is that part of the molecule that essentially gives the molecule its colour and may contribute to other features of the dye such as its lightfastness. As encountered in most of the other application classes of textile dyes, these chromogens typically belong to the azo, carbonyl or phthalocyanine chemical classes. Specific examples are used to illustrate this feature later in this chapter. Reactive dyes are required to be water — soluble for their application to the textile fibres, and they invariably contain for this purpose one or more ionic groups, most commonly the sulfonate group as the sodium salt. They are thus anionic in nature and, as such, show many of the chemical features of acid dyes for protein fibres (Chapter 7). Most commonly, the water-solubilising group is located in the chromogenic part of the reactive dye molecule, although on occasions it is part of the fibre-reactive group. The essential structural characteristic of a reactive dye is a functional group that is capable of reacting chemically with the fibre. This feature is, for obvious reasons, termed the fibre — reactive group and the organic chemistry underlying the reaction of these groups with functionality on the fibre forms a substantial part of this chapter. Commonly the term bridging group is used to identify the group of atoms which is used to link the chromogenic part of the molecule to the fibre-reactive group. In many dyes, the bridging group is the amino (-NH-) group, often for reasons of synthetic convenience. There are,
|
|
however, certain types of reactive dyes in which no obvious bridging functionality may be clearly identified.
 7 ноября, 2015
7 ноября, 2015  Pokraskin
Pokraskin 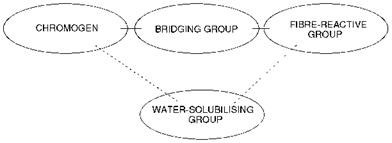
 Опубликовано в рубрике
Опубликовано в рубрике 