Cellulosic fibres are natural fibres derived from plant sources. The most important cellulosic fibres are cotton, viscose, linen, jute, hemp and flax. The principal component of the cotton fibre is cellulose, the structure of which is shown in Figure 7.4. Cotton is in fact almost pure cellulose (up to 95%). Cellulose is a polysaccharide. It is a high molecular weight polymer consisting of long chains of repeating glucose units, with up to around 1300 such units in each molecule. Cellulose has a fairly open structure, which allows large dye molecules to penetrate relatively easily into the fibre. Each glucose unit contains three hydroxy groups, two of which are secondary and one primary, and these give the cellulose molecule a considerable degree of polar character. The presence of the hydroxy groups is of considerable importance in the dyeing of cotton. For example, the ability of the hydroxy groups to form intermolecular hydrogen bonds is thought to be of some importance in direct dyeing, while reactive dyeing (Chapter 8) involves a chemical reaction of the hydroxy groups with the dye to form dye-fibre covalent bonds. The tendency of the hydroxy groups to ionise to a certain extent (to — O) means that the fibres can carry a small negative charge. There are a larger number of application classes of dyes that may be used to dye cellulosic fibres such as cotton than for any other fibre. These application classes include direct, vat, sulfur, azoic and reactive dyes. The structural features of two of the most important, direct and vat dyes are considered in this section while the discussion of reactive dyes is continued separately in Chapter 8.
|
|
An outline of the chemistry of sulfur dyes, which is not well established, is presented in Chapter 6.
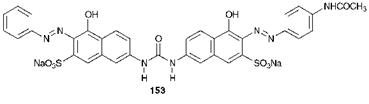
Direct dyes are a long-established class of dyes for cellulosic fibres. They derive their name historically from the fact that they were the first application class to be developed that could be applied directly to these fibres without the need for a fixation process such as mordanting. In some ways, direct dye molecules are structurally similar to acid dye molecules used for protein fibres. For example, they are anionic dyes as a result of the presence of sulfonate ( — SO3) groups. However, the role of the sulfonate groups in the case of direct dyes is simply to provide water solubility. In contrast to the acid dyeing of protein fibres, ionic attraction to the fibre is not involved in the direct dyeing of cellulosic fibres. In fact, the anionic nature of the dyes can reduce affinity for the fibres because cellulosic fibres may carry a small negative charge. For this reason, usually only as many sulfonate groups as are required to give adequate solubility in water are present in direct dyes and, in addition, the groups are distributed evenly throughout the molecules. Arguably the most important features of direct dye molecules which influence their application properties are their size and shape. They are, in general, large molecules and in shape they are long, narrow and planar. Direct dyes show affinity for cellulose by a combination of van der Waals’, dipolar and hydrogen-bonding intermolecular forces. Individually, these forces are rather weak. The long, thin and flat geometry of the molecules is essential to allow the dye molecules to align with the long polymeric cellulose fibre molecules and hence to maximise the overall effect of the combined set of intermolecular forces. Chemically, direct dyes, of which compound 153 (C. I. Direct Orange 25) is a typical example, are almost invariably azo dyes, commonly containing two or more azo groups. The long, flat, linear shape of compound 153 allows groups such as the — OH, — NHCO (amide), and — N-N — groups in principle to form hydrogen bonds with OH groups on cellulose as it lines up with the cellulose molecule. There are only two sulfonate groups in 153 and these are well separated. This is sufficient to give adequate water solubility for their application. Also, it may be argued that the sulfonate groups are on the opposite side of the molecule from groups which may be participating in
|
|
hydrogen bonding with the fibre and this means that they will be oriented away from the cellulose molecule, thus minimising any negative charge repulsion effects
Direct dyes, in comparison particularly with vat dyes and reactive dyes, provide only moderate washfastness. They are, however, inexpensive and are therefore the dyes of choice for applications, such as the coloration of paper, where cost is of prime concern and fastness to wet treatments is of lesser importance. For textile applications, the washfastness may in certain cases be improved by certain chemical aftertreatments. For example, a group of products referred to as direct and developed dyes contain free amino (-NH2) groups. Treatment of the dyed fabric with aqueous sodium nitrite under acidic conditions results in diazotisation of these groups and the resulting diazonium salts may be reacted with a variety of coupling components, such as 1-naphthol. The larger azo dye molecule, which is thus formed, is more strongly attracted to the fibre and less soluble in water, both features leading to improved washfastness. Alternatively, in a process referred to as after-coppering, some o, o’-dihydroxyazo direct dyes may be treated with copper(ii) salts to form square planar metal complexes which also show improved washfastness properties.
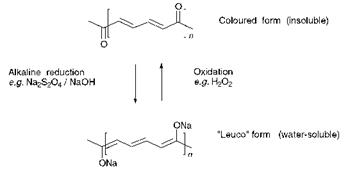
Vat dyes are a group of totally water-insoluble dyes and in this respect they are essentially pigments. In fact, some vat dyes, after conversion into a suitable physical form, may be used as pigments (Chapters 4 and 9). For application to cellulosic fibres, vat dyes are initially converted into a water-soluble ‘leuco’ form by an alkaline reduction process. Commonly, this conversion is carried out using a reducing agent such as sodium dithionite (Na2S2O4) in the presence of sodium hydroxide. In the leuco form, the dyes are taken up by the cellulosic fibre. Subsequently, they are converted back by an oxidation reaction to the insoluble pigment. Hydrogen peroxide is commonly used as the oxidising agent for this process although atmospheric oxygen can also effect the oxidation under appropriate conditions. The pigment or molecular aggregate thus produced becomes trapped mechanically within the fibre and its insolubility gives rise to the excellent washfastness properties which are characteristic of vat dyes. The vat dyeing process is usually completed with a high temperature aqueous surfactant treatment, or ‘soaping’, which enhances the molecular aggregation process and develops crystallinity. The chemistry of the reversible reduction-oxidation process involved in vat dyeing, which is illustrated in Figure 1.5, requires the presence in the vat dye of two carbonyl groups linked via a conjugated system. The carbonyl (C-O) groups are reduced under alkaline conditions to enolate ( — O~Na+) groups which give the leuco form water solubility.
Vat dyes are thus exclusively of the carbonyl chemical class. Dyes of
|
Figure 7.5 The chemistry of vat dyeing |
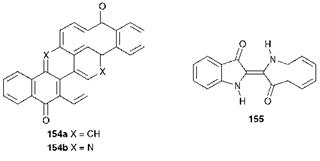
other chemical classes, including azo dyes, are generally inappropriate as vat dyes because they undergo a reduction that cannot be reversed. Vat dyes are generally large planar molecules, often containing multiple ring systems, to provide the leuco form of the dyes with some affinity for the cellulosic fibre as a result of van der Waals’ and dipolar forces. There is usually a notable absence of other functional groups in the molecules, because such groups can be sensitive to the reduction and oxidation reactions, although halogen substituents (Cl, Br) are encountered on occasions. Pyranthrone (154a, C. I. Vat Orange 9) and flavanthrone, (154b, C. I. Vat Yellow 1) provide examples respectively of carbocyclic and heterocyclic anthraquinone vat dyes (these carbonyl dyes are also discussed in Chapter 4). Indigo (155) is a further example of a long — established vat dye used commonly in the dyeing of denim fabric.
|
|
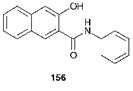
Azoic dyeing of cellulosic fibres is a process that is used only to a small extent today. In this process, an azo pigment is formed by chemical reaction within the fibre. The cotton fibres are first impregnated with an appropriate coupling component such as the anilide of 3-hydroxy-2- naphthoic acid, 156, under aqueous alkaline conditions. The fibre is then treated with a solution of a stabilised diazonium salt, in which the counter-anion is tetrafluoroborate (BF4~) or tetrachlorozincate (ZnCl42~), to form the insoluble azo pigment aggregates, which are trapped mechanically within the cotton fibres.
|
|
 31 октября, 2015
31 октября, 2015  Pokraskin
Pokraskin 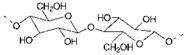
 Опубликовано в рубрике
Опубликовано в рубрике 