The nitro group is commonly encountered as a substituent in dyes and pigments of most chemical classes, but it acts as the essential chromo — phore in only a few dyes. Nitro dyes are a small group of dyes of some importance as disperse dyes for polyester and as semi-permanent hair dyes. Picric acid, 139, was historically the first nitro dye, although it was
never really commercially important due to its poor dyeing properties, its toxicity and its potential explosive properties (Chapter 1). The nitro dyes used today have relatively simple nitrodiphenylamine structures. Some examples of nitro dyes are shown in Figure 6.7. They contain at least one nitro (NO2) group as the chromophore and electron acceptor and the electron-releasing amino group completes the donor-acceptor system. Nitro dyes are capable of providing bright yellow, orange and red shades, but the colours are amongst the weakest provided by the common commercial chromophores. Nitrodiphenylamines are nevertheless of some importance as yellow disperse dyes, such as C. I. Disperse Yellow 42 (141), because of their low cost and their good lightfastness. The good lightfastness of these dyes is attributed to the intramolecular hydrogen bonding between the o-nitro group and the amino group while the electron-withdrawing (nitro or sulfonamide) group in the para-position is also important as it gives rise to an increase in tinctorial strength. A wider range of hues is provided by some nitro hair dyes, such as compounds 142, which is red, and 143 which is violet.
The synthesis of nitro dyes is relatively simple, a feature which accounts to a certain extent for their low cost. The synthesis, illustrated in Scheme 6.5 for compounds 140 and 141, generally involves a nucleophilic substitution reaction between an aromatic amine and a chloronitroaromatic compound. The synthesis of C. I. Disperse Yellow 14 (140) involves the reaction of aniline with 1-chloro-2,4-dinitroaniline while compound 141 is prepared by reacting aniline (2 mol) with compound 144 (1 mol).
|
Scheme 6.5 Synthesis of nitro dyes 140 and 141 |
 23 октября, 2015
23 октября, 2015  Pokraskin
Pokraskin 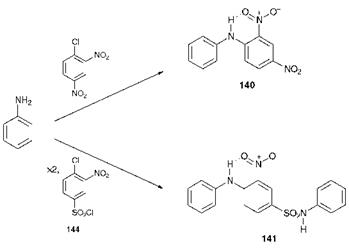
 Опубликовано в рубрике
Опубликовано в рубрике 