Polyene and polymethine dyes are two structurally related groups of dyes which contain as their essential structural feature one or more methine (-CH-) groups. Polyene dyes contain a series of conjugated double bonds, usually terminating in aliphatic or alicyclic groups. They owe their colour therefore simply to the presence of the conjugated system. In polymethine dyes, electron-donor and electron-acceptor groups terminate either end of the polymethine chain, so that they may be considered as typical donor-acceptor dyes.
The best-known group of polyene dyes is the carotenoids, which are widely encountered natural colorants. ^-Carotene (104) is given as an
|
|
important example. This dye is a hydrocarbon that contains eleven conjugated double bonds, which illustrates the length of chain that is necessary to shift the absorption from the UV into the visible range. Dye 104 shows absorption maxima at 450 and 478 nm. Naturally occurring carotenoids not only provide the attractive yellow, orange and red colours of many plants, but also serve important biochemical functions. For example, they may protect cells and organisms against some of the harmful effects of exposure to light as a result of their action as efficient deactivators of singlet oxygen, and they are also important in some biological energy transfer processes. Nutritionists have always encouraged us to include significant quantities of fresh fruit and vegetables in our diet. Special emphasis has been added to this advice in recent years as evidence has emerged for the potential therapeutic benefits of carotenoids and other related natural colorants, including the possibility that they may inhibit tumour development, no doubt related to their ability to quench singlet oxygen. Another carotenoid which is of some considerable functional importance is retinal, which is the chromogenic part of the molecules of rhodopsin and iodopsin, the pigments in the eye which are responsible for colour vision (Chapter 2). There are no true synthetic polyene dyes of any real commercial importance. However, the phthalocyanines (Chapter 5) may, in a sense, be considered, structurally, as aza analogues of a cyclic polyene system.
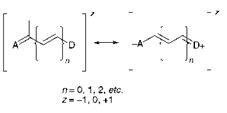
In polymethine dyes, electron donor (D) and acceptor (A) groups terminate the polymethine chain as illustrated by the general structure given in Figure 6.1. They embrace a wide variety of structural types which may be subdivided into three broad categories as either cationic (z = + 1), anionic (z = — 1) or neutral (z = 0) types, depending on the precise nature of A and D. Dyes of a similar structure in which one or more of the methine carbon atoms are replaced by aza nitrogen atoms are also conveniently considered as polymethines. Polymethine dyes are capable of providing a wide range of bright, intense colours but, in general, they have tended to show rather poor fastness properties compared with other chemical classes. This feature has limited their use on textiles, where they are restricted mainly to some disperse dyes for polyester and cationic dyes for acrylic fibres and basic dyes They are essentially of no industrial consequence as pigments. They are, however, used in the
|
Figure 6.1 General structure of polymethine dyes |
colour photographic industry, and they have been extensively studied because of fundamental theoretical interest.
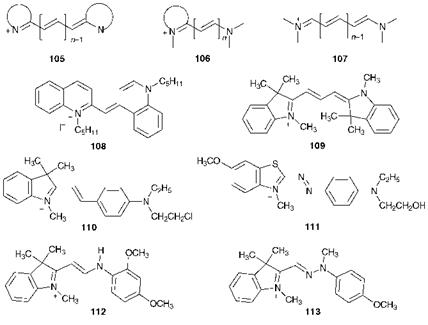
The most important group of cationic polymethine dyes contains nitrogen atoms in both the D and A groups. These are further classified as cyanines, 105, hemicyanines, 106, or streptocyanines, 107, depending on whether both, one or neither of the nitrogen atoms is contained in a heterocyclic ring, as illustrated in Figure 6.2. The history of this type of dye dates from 1856, the same year in which Perkin discovered Mauveine (Chapter 1). In that year, Williams discovered that the reaction of crude quinoline, which fortuitously contained substantial quantities of 4- methylquinoline, with isopentyl iodide in the presence of alkali gave a blue dye which he named Cyanine. This dye was eventually identified as having the structure 108. A range of cyanine dyes was subsequently prepared, but these early products proved to be of little use for textiles because of their poor lightfastness. However, considerable interest in their chemistry led ultimately to the discovery that certain of the dyes had photosensitising properties and this gave rise to their application in colour photography. A new era for cationic polymethine dyes was heralded by the introduction in the 1950s of polyacrylonitrile fibres. It was found that such dyes when applied to acrylic fibres containing anionic sites were capable of giving bright, strong shades with good fastness properties (Chapter 7). Examples of dyes of this type are the cyanine C. I. Basic Red 12, 109, the hemicyanine C. I. Basic Violet 7, 110, the dia — zahemicyanine C. I. Basic Blue 41,111 (which may also be considered as a member of the azo dye class), the azacyanine C. I. Basic Yellow 11, 112, and the diazacyanine C. I. Basic Yellow 28,113. In this series, the cyanines and their aza analogues generally give yellow through to red dyes, while the corresponding hemicyanines are more bathochromic, giving reds, violets and blues.
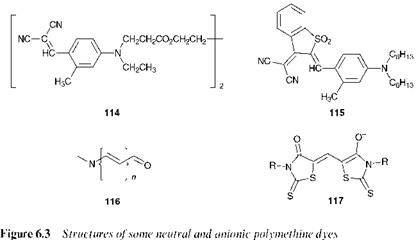
The structures of a number of neutral and anionic polymethine dyes are illustrated in Figure 6.3. There are many types of neutral poly — methines, utilising a wide range of electron donor and acceptor groups. For example, C. I. Disperse Yellow 99 (114) and C. I. Disperse Blue 354
|
Figure 6.2 Structures of some cationic polymethine dyes |
(115) are important dyes for polyester (Chapter 7). Merocyanines in which the amino group is the donor and the carbonyl group is the acceptor, as represented by the general structure 116, are well known. Because of their generally inferior lightfastness properties they are not used on textiles, but find wider use in colour photography. Merocyanines are also formed when certain colourless photochromic compounds, notably the spirooxazines, are irradiated with light. The reversible colour change given by these compounds may be used in a range of applications, for example in ophthalmics, security printing and optical data storage (Chapter 10). Anionic polymethines, such as the oxonol, 117, are usually rather unstable components and so they have not been so extensively investigated.
Symmetrical cyanine dyes, because of the resonance shown in Figure 6.4 (in which the two contributing structures are exactly equivalent), are completely symmetrical molecules. X-ray crystal structure determinations and NMR spectroscopic analysis have demonstrated that the dyes are essentially planar and that the carbon-carbon bond lengths in the polymethine chain are uniform. The colour of cyanine dyes depends mainly on the nature of the terminal groups and on the length of the polymethine chain. The bathochromicity of the dyes is found to increase
|
|
|
Figure 6.4 Valence-bond (resonance) approach to cyanine dyes |
with the electron-releasing power of the terminal donor group. More bathochromic dyes are also obtained by incorporating the terminal group into a heterocyclic ring system and by extending the conjugation. Cyanine dyes display a highly allowed HOMO LUMO transition, and hence they show high molar extinction coefficients, i. e. high colour strength. Another important feature of cyanine dyes is the narrowness of the absorption bands, which means that they are exceptionally bright colours. One factor which influences the absorption bandwidth of a dye is how closely the geometry of the first excited state of the molecule resembles that of the ground state. In the case of cyanine dyes these two states exhibit very similar geometry and hence the absorption bands are narrow. The results of molecular orbital calculations, for example using the PPP-MO approach, are in good agreement with the experimental spectral data for a wide range of polymethine dyes. In addition, the calculations confirm that there is no major redistribution of л-electron charge densities or л-bond orders on excitation.
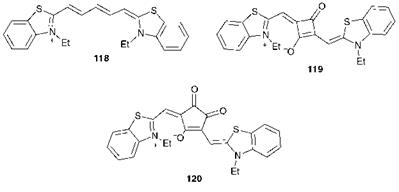
There has been some interest in extending the absorption range of cyanine dyes to longer wavelengths into the near-infrared region of the spectrum. Consideration of the spectral data for thiazole derivatives 118-120 is of some interest in this respect. Cyanine dye 118 shows the characteristic visible absorption spectrum for a dye of this type, giving a
|
|
narrow band with a Ятах value of 651 nm in acetonitrile. As the length of the conjugated polymethine chain of cyanine dye 118 is extended further, the absorption band is shifted bathochromically by about 100 nm for each additional — CH=CH— group. However, the absorption curves become increasingly broad as the chain is extended and there is a consequent reduction in the molar extinction coefficient. It has been suggested that this may be due to trans=cis isomerism, which becomes more facile as the length of the chain increases, and also to an increase in the participation of higher vibrational states of the first excited state as the molecular flexibility is increased. As an alternative to simple extension of the conjugation some structurally related systems have been investigated. The squarylium dye 119 absorbs at 663 nm and gives a considerably narrower bandwidth than compound 118, while the croconium derivative 120 gives a narrow absorption band at 771 nm. Dyes of these structural types are of some interest for their potential to provide intense narrow absorption bands in the near-infrared region with minimal absorption in the visible region (Chapter 10).
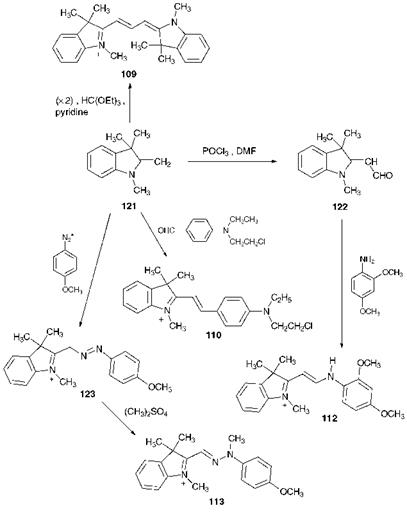
The strategies used in the synthesis of polymethine dyes are illustrated for a series of indoline derivatives in Scheme 6.1. There is an even wider range of synthetic routes to polymethine dyes than is described here, but they are based for the most part on a similar set of principles. The starting material for the synthesis of this group of polymethine dyes is invariably 2-methylene-1,3,3-trimethylindolenine (121), known universally as Fischer’s base. As illustrated in the scheme, compound 121 may be converted by formylation using phosphoryl chloride and dimethylformamide into compound 122, referred to as Fischer’s aldehyde, which is also a useful starting material for this series of polymethine dyes. When compound 121 (2 mol) is heated with triethylorthoformate (1 mol) in the presence of a base such as pyridine, the symmetrical cyanine dye, C. I. Basic Red 12 109 is formed. The synthesis of some hemicyanines may be achieved by
|
Scheme 6.1 Synthetic approach to some indolenine-based polymethine dyes |
heating Fischer’s base with an aldehyde, as illustrated for the case of C. I. Basic Violet 7, 110. The azacyanine C. I. Basic Yellow 11 (112) is synthesised by the condensation reaction of Fischer’s aldehyde 122 with 2,4-dimethoxyaniline. In the synthesis of diazacyanine dye C. I. Basic Yellow 28 (113), an important golden yellow dye for acrylic fibres, Fischer’s base 121 is treated with 4-methoxybenzenediazonium chloride and undergoes an azo coupling reaction at the methylene group to give the azo dye 123. Methylation of this dye with dimethyl sulfate gives the diazacyanine dye 113.
 19 октября, 2015
19 октября, 2015  Pokraskin
Pokraskin 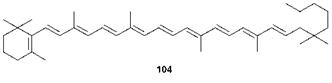

 Опубликовано в рубрике
Опубликовано в рубрике 