The synthesis of anthraquinone colorants may effectively be envisaged as involving two stages. The first stage involves the construction of the anthraquinone ring system and in the second phase the anthraquinone nucleus is elaborated to produce the desired structure. Frequently the latter involves substitution reactions, but group interconversion and further cyclisation reactions may also be employed. Although the chemistry of the synthesis of most anthraquinone dyes and pigments is long established, some of the mechanistic detail of the individual reactions remains unexplained.
There are two principal ways of constructing the anthraquinone system which are successful industrially. The first of these is the oxidation of anthracenes and the second involves a Friedel-Crafts acylation route. Anthracene (73), a readily available raw material since it is a major constituent of coal tar, may be oxidised to give anthraquinone (52) in high yield as illustrated in Scheme 4.2. The most important oxidising agents used in this process are sodium dichromate and sulfuric acid (chromic acid) or nitric acid. This route is of considerable importance for the synthesis of the parent anthraquinone (52), but it is of much less importance for the direct synthesis of substituted anthraquinones. The main reasons for this are that there are few substituted anthracenes readily available as starting materials and also because many substituents would be susceptible to the strongly oxidising conditions used.
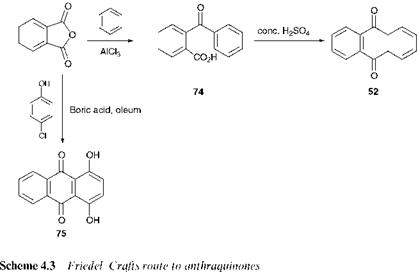
An alternative route to anthraquinone, which involves Friedel-Crafts acylation, is illustrated in Scheme 4.3. This route uses benzene and phthalic anhydride as starting materials. In the presence of aluminium(iii) chloride, a Lewis acid catalyst, these compounds react to form 2-benzoyl — benzene-1-carboxylic acid, 74. The intermediate 74 is then heated with concentrated sulfuric acid under which conditions cyclisation to anthraquinone 52 takes place. Both stages of this reaction sequence involve Friedel-Crafts acylation reactions. In the first stage the reaction is intermolecular, while the second step in which cyclisation takes place, involves an intramolecular reaction. In contrast to the oxidation route, the Friedel-Crafts route offers considerable versatility. A range of substituted
|
|
|
Scheme 4.2 Oxidation of anthracene
|
benzene derivatives and phthalic anhydrides may be brought together as starting materials, leading to a wide range of substituted anthraquinones. For example, the use of toluene rather than benzene leads directly to 2-methylanthraquinone. A further industrially important example is also illustrated in Scheme 4.3. Starting from 4-chlorophenol and phthalic anhydride, two inexpensive and readily available starting materials, 1,4- dihydroxyanthraquinone, quinizarin, (75), an important intermediate in the synthesis of a number of anthraquinone dyes, may be synthesised efficiently in a ‘one-pot’ reaction. Boric acid in oleum is used as the catalyst and solvent system in this case. During the course of the reaction in which the anthraquinone nucleus is formed, a hydroxy group replaces the chlorine atom. The detailed mechanism by which this takes place has not been fully established.
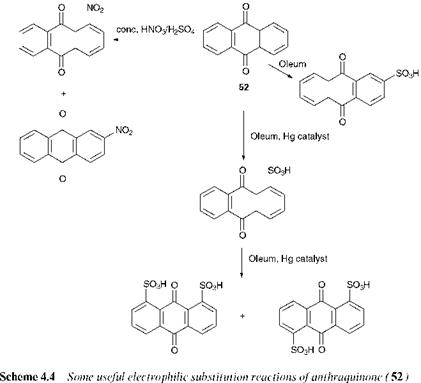
The outer rings of the anthraquinone molecule (52) are aromatic in nature and as such are capable of undergoing substitution reactions. The reactivity of the rings towards substitution is determined by the fact that
|
|
they are attached to two electron-withdrawing carbonyl groups. The presence of these groups deactivate the aromatic rings towards electrophilic substitution. Nevertheless, using reasonably vigorous conditions, anthraquinone may be induced to undergo electrophilic substitution reactions, notably nitration and sulfonation. Scheme 4.4 illustrates a series of electrophilic substitution reactions of anthraquinone. Nitration of anthraquinone with mixed concentrated nitric and sulfuric acids leads to a mixture of the 1- and 2-nitroanthraquinones which is difficult to separate and so this process is of limited use. However, nitration of some substituted anthraquinones, particularly some hydroxy derivatives, can give more useful products. Sulfonation of anthraquinone requires the use of oleum at elevated temperatures, and under these conditions the reaction leads mainly to the 2-sulfonic acid. However, in the presence of a mercury(ii) salt as catalyst, the 1-isomer, a much more useful dye intermediate, becomes the principal product. A probable explanation for these observations is that, in the absence of a catalyst, the 2-position is preferred sterically. In the presence of the catalyst, it has been proposed that mercuration takes place first preferentially at the 1-position, and the mercury is displaced subsequently by the electrophile responsible for
|
Scheme 4.5 Reaction of bromamine acid with aromatic amines |
|
Scheme 4.6 Formation of 1,4-diaminoanthraquinonesfrom quinizarin |
sulfonation. Disulfonation of anthraquinone in the presence of mercury salts leads to a mixture of the 1,5- and 1,8-disulfonic acids, which are easily separated and these are also useful dye intermediates.
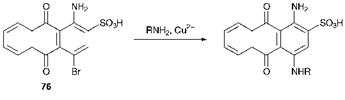
Nucleophilic substitution reactions, to which the aromatic rings are activated by the presence of the carbonyl groups, are commonly used in the elaboration of the anthraquinone nucleus, particularly for the introduction of hydroxy and amino groups. Commonly these substitution reactions are catalysed by either boric acid or by transition metal ions. As an example, amino and hydroxy groups may be introduced into the anthraquinone system by nucleophilic displacement of sulfonic acid groups. Another example of an industrially useful nucleophilic substitution is the reaction of 1-amino-4-bromoanthraquinone-2-sulfonic acid (bromamine acid) (76) with aromatic amines, as shown in Scheme 4.5, to give a series of useful water-soluble blue dyes. The displacement of bromine in these reactions is catalysed markedly by the presence of copper(ii) ions.
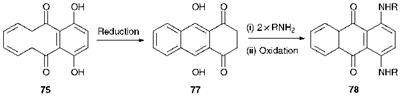
An important route to 1,4-diaminoanthraquinones, represented by structure 78, is illustrated in Scheme 4.6. Quinizarin (75) is first reduced to leucoquinizarin, which has been shown to exist as the diketo structure 77. Condensation of compound 77 with two moles of an amine, followed by oxidation leads to the diaminoanthraquinone 78. Boric acid is a useful catalyst for this reaction, particularly when less basic amines are used.
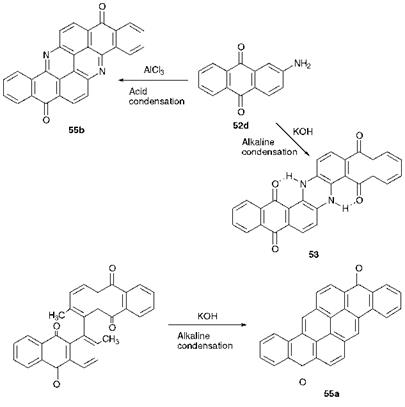
The syntheses of three polycyclic anthraquinones, indanthrone (53), pyranthrone (55a) and flavanthrone (55b), are illustrated in Scheme 4.7. In spite of the structural complexity of the products, the syntheses of these types of compound are often quite straightforward, involving, for
|
Scheme 4.7 Syntheses of the polycyclic anthraquinones indanthrone (53), pyranthrone (55a) and flavanthrone (55b) |
example, condensation or oxidative cyclisation reactions. For example, the blue vat dye indanthrone (53) is prepared by fusion of 2-aminoan — thraquinone (52d) with either sodium or potassium hydroxide at around 220 °C. Curiously, the same dye can be prepared from 1-aminoan — thraquinone (52c) in a similar way, although the 2-isomer is the usual industrial starting material. Pyranthrone (55a) is also prepared by an alkaline fusion process, starting in this case from 2,2′-dimethyl-1,1′-dia — nthraquinonyl. The methyl groups in this molecule are sufficiently acidic as a result of activation by the carbonyl groups to be ionised and subsequently give rise to cyclisation by a reaction analogous to an aldol condensation. Flavanthrone (55b) may also be prepared by a condensation reaction starting from 2-aminoanthraquinone (52d). The yellow vat dye is obtained from fusion of 52d with alkali at temperatures of around 300 °C or by acid-catalysed condensation, for example using alumin — ium(iii) chloride. However, its industrial manufacture usually involves a
|
|
similar condensation process, either acid or base catalysed, starting from 1-chloro-2-acetylaminoanthraquinone.
 30 сентября, 2015
30 сентября, 2015  Pokraskin
Pokraskin 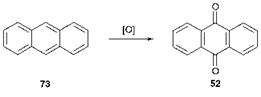
 Опубликовано в рубрике
Опубликовано в рубрике 