The most obvious requirement of a dye or pigment to be useful in its applications is that it must have an appropriate colour. Of the many ways in which light can interact with objects, the two most important from the point of view of their influence on colour are absorption and scattering. Absorption is the process by which radiant energy is utilised to raise molecules in the object to higher energy states. Scattering is the interaction by which light is re-directed as a result of multiple refractions and reflections. In general, if only absorption is involved when light interacts with an object, then the object will appear transparent as the light that is not absorbed is transmitted through the object. If there are scattering centres present, the object will appear either translucent or opaque, depending on the degree of scattering, as light is reflected back to the observer.
Electronic spectroscopy, often referred to as UV/visible spectroscopy, is a useful instrumental technique for characterising the colours of dyes and pigments. These spectra may be obtained from appropriate samples either in transmission (absorption) or reflection mode. UV/visible absorption spectra of dyes in solution, such as that illustrated in Figure 2.3, provide important information to enable relationships between the colour and the molecular structure of the dyes to be developed.
A dye in solution owes its colour to the selective absorption by dye molecules of certain wavelengths of visible light. The remaining wavelengths of light are transmitted, thus giving rise to the observed colour. The absorption of visible light energy by the molecule promotes electrons in the molecule from a low energy state, or ground state, to a higher energy state, or excited state. The dye molecule is therefore said to undergo an electronic transition during this excitation process. The
|
Figure 2.3 U V/visible absorption spectrum of a typical red dye in solution |
energy difference, AE, between the electronic ground state and the electronic excited state is given by Planck’s relationship
AE = hv
where h is a constant (Planck’s constant) and v is the frequency of light absorbed. Alternatively, the relationship may be expressed as
AE = hc/k
where c is the velocity of light (also a constant) and k is the wavelength of light absorbed. Thus there is an inverse relationship between the energy difference between the ground and excited states of the dye and the wavelength of light that it absorbs. As a consequence, for example, a yellow dye, which absorbs short wavelength (blue) light, requires a higher excitation energy than, say, a red dye which absorbs longer wavelength (bluish-green) light (Table 2.1).
There are a number of ways of describing in scientific terms the characteristics of a particular colour. One method which is especially useful for the purposes of relating the colour of a dye to its UV/visible spectrum in solution is to define the colour in terms of the three attributes: hue (or shade), strength (or intensity) and brightness. The hue of a dye is determined essentially by the absorbed wavelengths of light, and so it may be characterised to a reasonable approximation, at least in those cases where there is a single visible absorption band, by the kmax value obtained from the UV/visible spectrum. A shift of the absorption band towards longer wavelengths (i. e. a change of hue in the direction yellow oranges red violets blue green), for example as a result of a structural change in a dye molecule, is referred to as a bathochromic shift. The reverse effect, a shift towards shorter absorbed wavelengths, is described as a hypsochromic shift.
A useful measure of the strength or intensity of the colour of a dye is given by the molar extinction coefficient (e) at its kmax value. This quantity may be obtained from the UV/visible absorption spectrum of the dye by using the Beer-Lambert law, i. e.
A = ecl
where A is the absorbance of the dye at a particular wavelength, e is the molar extinction coefficient at that wavelength, c is the concentration of the dye and l is the path length of the cell (commonly 1 cm) used for measurement of the spectrum. The Beer-Lambert law is obeyed by most dyes in solution at low concentrations, although when dyes show molecular aggregation effects in solution, deviations from the law may be encountered. However, since the colour strength of a dye is more correctly related to the area under the absorption band, it is important to treat its relationship with the molar extinction coefficient as qualitative and dependent to a certain extent on the shape of the absorption curve.
The third attribute, brightness, may be described in various other ways, for example as brilliance or vividness. This characteristic of the colour depends on the absence of wavelengths of transmitted light other than those of the hue concerned. Electronic absorption bands of molecular compounds are not infinitely narrow because they are broadened by the superimposition of numerous vibrational energy levels on both the ground and excited electronic states. Brightness of colour is characterised, in terms of the UV/visible spectrum, by the shape of the absorption band. Dyes which exhibit bright colours show narrow absorption bands, whereas broad absorption bands are characteristic of dull colours, such as browns, navy blues and olive greens.
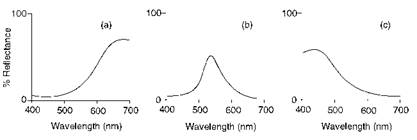
Visible reflectance spectroscopy is used routinely to measure the colour of opaque objects such as textile fabrics, paint films and plastics for purposes such as colour matching and dye and pigment recipe prediction. In many ways, this technique may be considered as complementary to the use of visible absorption spectroscopy for the measurement of transparent dye solutions. Reflectance spectra of typical red, green and blue surfaces are shown in Figure 2.4. The spectrum of the red surface, for example, shows low reflectance (high absorption) in the 400-500 nm (blue) and 500-600 nm (green) ranges, and high reflectance of the red wavelengths (600-700 nm).
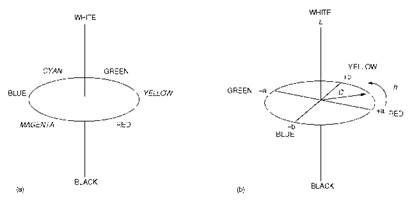
When colour is assessed on the basis of reflectance measurements, it is common to consider the three relevant attributes of perception of colour as hue, chroma (or saturation) which is the ‘colourfulness’ or richness of the colour, and lightness, which refers to the amount of reflected light. These three attributes may be described using the concept of colour space, which shows the relationships of colours to one another and which illustrates the three-dimensional nature of colour, as portrayed in Figure
|
Figure 2.4 Visible reflectance spectra of (a) red, (b) green and (c) blue surfaces |
|
Figure 2.5 (a)The concept of colour space; (b) LAB colour space |
2.5(a). The hue of a particular colour is represented in a colour circle. The three additive primaries, red, green and blue are equally spaced around the colour circle. The three subtractive primaries, yellow, magenta and cyan are located between the pairs of additive primaries from which they are obtained by mixing. The second attribute, chroma, increases with distance from the centre of the circle. The third attribute, lightness, requires a third dimension that is at right angles to the plane of the colour circle. The achromatic colours, white and black, are located at either extreme of the lightness scale. Mathematical approaches which make use of the concept of colour space for colour measurement and specification are now well known. In one of the most important of these approaches, the CIELAB equation for the measurement of colour differences makes use of the visually uniform LAB space, which is illustrated in Figure 2.5(b). Lightness, L and chroma, C, are quantified as illustrated on the diagram. Hue is described by the hue angle, h°. Colour measurement, and its mathematical basis, generally referred to as colour physics, is a well- developed and well-documented science and is not considered in further detail here.
 25 августа, 2015
25 августа, 2015  Pokraskin
Pokraskin 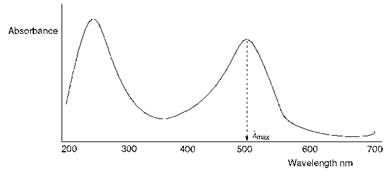
 Опубликовано в рубрике
Опубликовано в рубрике 