We only have to open our eyes and look around to observe how important a part colour plays in our everyday lives. Colour influences our moods and emotions and generally enhances the way in which we enjoy our surroundings. Our experience of colour emanates from a rich diversity of sources, both natural and synthetic. Natural colours are all around us, in the earth, the sky, the sea, animals and birds and in the vegetation, for example in the trees, leaves, grass and flowers. Colour is an important aspect in our enjoyment of the food we eat. In fact, we frequently judge the quality of meat products, fruit and vegetables by the richness of their colour. In addition, there is a myriad of examples of synthetic colours, products of the chemical manufacturing industry, which we tend to take so much for granted these days. These colours commonly serve a purely decorative or aesthetic purpose, but in some cases specific colours may be used to convey vital information, for example in traffic lights and colour- coded electrical cables. Synthetic colours are used in the clothes we wear, in paints, plastic articles, in a wide range of multicoloured printed material such as posters, magazines and newspapers, in photographs, cosmetics, ceramics, and on television and film. Colour is introduced into these materials using substances known as dyes and pigments. The essential difference between these two types of colorants is that dyes are soluble coloured compounds which are applied mainly to textile materials from solution in water, whereas pigments are insoluble compounds incorporated by a dispersion process into products such as paints, printing inks and plastics. The reader is directed to Chapter 2 of this book for a more detailed discussion of the distinction between dyes and pigments as colouring materials.
People have made use of colour since prehistoric times, for example in decorating their bodies, in colouring the furs and skins that they wore and in the paintings which adorned their cave dwellings. Of course, in those days the colours that were used were derived from natural resources. The dyes used to colour clothing were commonly extracted either from vegetable sources, including plants, trees, roots, seeds, nuts, fruit skins, berries and lichens, or from animal sources such as crushed insects and molluscs. The pigments for the paints were obtained from coloured minerals, such as ochre and haematite, which were dug from the earth, ground to a fine powder and mixed into a crude binder.
Synthetic colorants may also be described as having an ancient history, although this statement applies only to a range of pigments produced from basic applications of inorganic chemistry. These very early synthetic inorganic pigments have been manufactured and used in paints for many thousands of years. The ancient Egyptians were probably responsible for the development of the earliest synthetic pigments. The most notable products were Alexandra blue, a ground glass coloured with a copper ore, and Egyptian Blue, a mixed silicate of copper and calcium which has been identified in murals dating from around 1000 BC. Perhaps the oldest synthetic colorant still used extensively today is Prussian Blue, the structure of which has been established as iron(iii) hexacyanoferrate(ii). The manufacture of this blue inorganic pigment is much less ancient, dating originally from the middle of the 17th century, although this product pre-dates the origin of synthetic organic dyes and pigments by more than a century.
Synthetic textile dyes are exclusively organic compounds and, in relative historical terms, their origin is much more recent. Textile materials were coloured exclusively with the use of natural dyes until the mid-19th century. Since most of nature’s dyes are rather unstable, the dyeings produced in the very early days tended to be quite fugitive, for example to washing and light. Over the centuries, however, complex dyeing procedures using a selected range ofnatural dyes were developed which were capable of giving reasonably quality dyeing on textile fabrics. Since natural dyes generally have little direct affinity for textile materials, they were usually applied together with compounds known as mordants, which were effectively ‘fixing-agents’. Metal salts, for example of iron, tin, chromium, copper or aluminium, were the most commonly used mordants, and these functioned by forming complexes of the dyes within the fibre. These complexes were insoluble and hence more resistant to washing processes. As a result, these agents not only improved the fastness properties of the dyeing, but also in many instances were essential to develop the intensity and brightness of the colours produced by the natural dyes. Some natural organic materials such as tannic and tartaric acids could also be used as mordants. Among the most important of the natural dyes the use of which has been sustained over the centuries, is indigo 1a, a blue dye obtained from certain plants, for example from Indigofera tinctoria found in India, and from woad, a plant extract. A related product is Tyrian purple, whose principal constituent was 6,6′- dibromoindigo 1b. This was for many years a fashionable purple dye which was extracted from the glands of Murex brandaris, a shellfish found on the Mediterranean and Atlantic coasts. The most important of the natural red dyes was madder, a wood extract, the main constituent of which was alizarin, 1,2-dihydroxyanthraquinone (2). Alizarin provides a good example of the use of the mordanting process, since it readily forms metal complexes within fibres, notably with aluminium, which show more intense colours and an enhanced set of fastness properties.
|
|
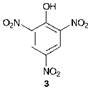
It may be argued that the first synthetic dye was picric acid 3, which was first prepared in the laboratory in 1771 by treating indigo with nitric acid. Much later, a more efficient synthetic route to picric acid from phenol as the starting material was developed. Picric acid was found to dye silk a bright greenish-yellow colour but it did not attain any real significance as a practical dye mainly because the dyeings obtained were of poor quality, especially in terms of lightfastness. However, it did find limited use at the time to shade indigo dyeings to give bright greens.
|
|
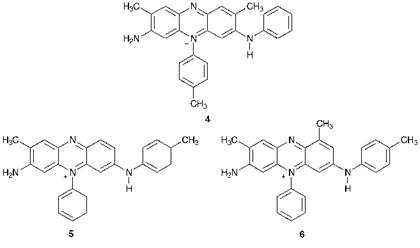
The foundation of the synthetic dye industry is universally attributed to William Henry Perkin on account of his discovery in 1856 of a purple dye which he originally gave the name Aniline Purple, but which was later to become known as Mauveine. Perkin was a young enthusiastic British organic chemist who was carrying out research aimed not initially at synthetic dyes but rather at developing a synthetic route to quinine, the antimalarial drug. His objective in one particular set of experiments was to prepare synthetic quinine from the oxidation of allyltoluidine, but his attempts to this end proved singularly unsuccessful, and, with hindsight, this is not too surprising in view of our current knowledge of the complex heteroalicyclic structure of quinine. As an extension of this research, he turned his attention to the reaction of the simplest aromatic amine, aniline, with the oxidising agent potassium dichromate. This reaction gave a black product which to many chemists might have seemed rather unpromising, but from which Perkin discovered that a low yield of a purple dye could be extracted with solvents. An evaluation of the dye in a silk dyeworks in Perth, Scotland, established that it could be used to dye silk a rich purple colour and give reasonable fastness properties. Perkin showed remarkable foresight in recognising the potential of his discovery. He took out a patent on the product and had the boldness to instigate the development of a large-scale manufacturing process. The dye was launched on the market in 1857. Since the manufacture required the development of large-scale industrial procedures for the manufacture of aniline from benzene via reduction of nitrobenzene, the real significance of Perkin’s venture was as the origin of the organic chemicals industry, which has evolved from this humble beginning to become a dominant feature of the industrial base of many of the world’s developed countries. For many years, the structure of Mauveine was reported erroneously as 4. It has been demonstrated only relatively recently from an analytical investigation of an original sample that the dye is a mixture and that the structures of the principal constituents are in fact compounds 5 and 6. The presence of the methyl groups, which are an essential feature of the product, demonstrate how fortuitous it was that Perkin’s crude aniline contained significant quantities of the toluidines. Compound 5, the major component of the dye, is derived from two molecules of aniline, one of p-toluidine and one of o-toluidine, while compound 6 is formed from one molecule of aniline, one of p-toluidine and two molecules of o-toluidine.
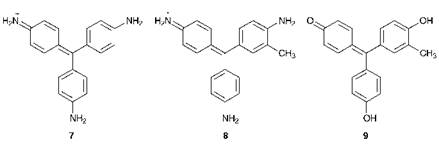
During the several years following the discovery of Mauveine, research activity in dye chemistry intensified, especially in Britain, Germany, and France. For the most part, chemists concentrated on aniline as the starting material, adopting a largely empirical approach to its conversion into coloured compounds, and this resulted in the discovery of several other synthetic textile dyes with commercial potential. In fact the term ‘Aniline Dyes’ was for many decades synonymous with synthetic dyes. Most notable among the initial discoveries were the triphenylmethine dyes, the first important commercial example of which was Magenta, introduced in 1859. Magenta was first prepared by the oxidation of crude aniline (containing variable quantities of the toluidines) with tin(iv) chloride. The dye contains two principal constituents, rosaniline 7 and
 |
homorosaniline 8, the central carbon atom being derived from the methyl group of p-toluidine. A structurally related dye, rosolic acid had been prepared in the laboratory in 1834 by the oxidation of crude phenol, and therefore may be considered as one of the earliest synthetic dyes, although its commercial manufacture was not attempted until the 1860s. Structure 9 has been suggested for rosolic acid, although it seems likely that other components were present. A wide range of new triphenylmethine dyes soon emerged and these proved quite quickly to be superior in properties and were more economic compared with Mauveine, the production of which ceased after about ten years. All of these dyes, including Mauveine, may be considered as examples of the arylcarbonium ion chemical class of dyes, many of which are still of current importance (see Chapter 6).
|
Undoubtedly the most significant discovery in colour chemistry in the |
‘post-Mauveine’ period was due to the work of Peter Griess, which provided the foundation for the development of the chemistry of azo dyes and pigments. In 1858, Griess demonstrated that the treatment of an aromatic amine with nitrous acid gave rise to an unstable salt (a dia — zonium salt) which could be used to prepare highly coloured compounds.
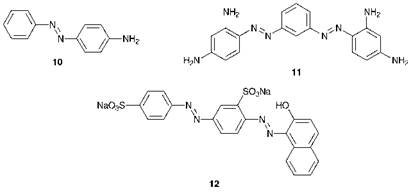
The earliest azo dyes were prepared by treatment of aromatic amines with a half-equivalent of nitrous acid, so that half of the amine was diazotised and the remainder acted as the coupling component in the formation of the azo compound. The first commercial azo dye was 4-aminoazoben — zene (10), Aniline Yellow, prepared in this way from aniline, although it proved to have quite poor dyeing properties. A much more successful commercial product was Bismarck Brown, (originally named Manchester Brown) — actually a mixture of compounds, the principal constituent of which is compound 11. This dye was obtained directly from m — phenylenediamine as the starting material and was introduced commercially in 1861. The true value of azo dyes eventually emerged when it was demonstrated that different diazo and coupling components could be used, thus extending dramatically the range of coloured compounds which could be prepared. The first commercial azo dye of this type was Chrysoidine which was derived from reaction of diazotised aniline with m-phenylenediamine and was introduced to the market in 1876. This was followed soon after by a series of orange dyes (Orange I, II, III and IV) which were prepared by reacting diazotised sulfanilic acid (4-aminoben — zene-1-sulfonic acid) respectively with 1-naphthol, 2-naphthol, N, N — dimethylaniline and diphenylamine. In 1879, Biebrich Scarlet, 12, the first commercial disazo dye to be prepared from separate diazo and coupling components, was introduced. History has demonstrated that azo dyes were to emerge as by far the most important chemical class of dyes and pigments, dominating most applications (see Chapter 3). It was becoming apparent that the synthetic textile dyes which were being developed were cheaper, easier to apply and were capable of providing better colour and technical performance than the range of natural dyes applied by traditional methods. As a consequence, within 50 years of Perkin’s initial discovery, around 90% of textile dyes were synthetic rather than natural and azo dyes had emerged as the dominant chemical type.
|
|
Towards the end of the 19th century, a range of organic pigments was also being developed commercially, particularly for paint applications. These were found to provide brighter, more intense colours than the inorganic pigments which had been in use for many years. Initially, most of these organic pigments were obtained from established water-soluble textile dyes. Anionic dyes were rendered insoluble by precipitation onto inert colourless inorganic substrates such as alumina and barium sulfate while, alternatively, basic dyes were treated with tannin or antimony potassium tartrate to give insoluble pigments. Such products were commonly referred to as ‘lakes’. Their introduction was followed soon after by the development of a group of yellow and red azo pigments, such as the Hansa Yellows and ^-naphthol reds, which did not contain substituents capable of salt formation. Many of these products are still of considerable importance today, and are referred to commonly as the classical azo pigments (see Chapter 9).
It is of interest and in a sense quite remarkable that, at the time of Perkin’s discovery of Mauveine, chemists had very little understanding of the principles of organic chemistry. As an example, even the structure of benzene, the simplest aromatic compound, was an unknown quantity. Kekule’s proposal concerning the cyclic structure of benzene in 1865 without doubt made one of the most significant contributions to the development of organic chemistry. It is certain that the commercial developments in synthetic colour chemistry which took place from that time onwards owed much to the coming of age of organic chemistry as a science. For example, the structures of some of the more important natural dyes, including indigo (1a) and alizarin (2), were deduced. In this period well before the advent of the modern range of instrumental analytical techniques which are now used routinely for structural analysis, these deductions usually arose from a painstaking investigation of the chemistry of the dyes, commonly involving a planned series of degradation experiments from which identifiable products could be isolated. Following the elucidation of the chemical structures of these natural dyes, a considerable amount of research effort was initiated which was devoted to devising efficient synthetic routes to these products. The synthetic routes which were developed for the manufacture of these dyes proved very quickly to be significantly more cost-effective than the traditional methods which involved extracting the dyes from natural sources and in addition gave the products more consistently and with better purity. At the same time, by exploring the chemistry of these natural dye systems, chemists were discovering a wide range of structurally related dyes which could be produced synthetically and which had excellent colour properties and technical performance. As a consequence, the field of carbonyl dye chemistry, and the anthraquinones in particular, had opened up and this group of dyes remains for most textile applications the second most important chemical class, after the azo dyes, in use today (see Chapter 4).
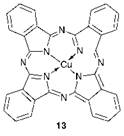
In the first half of the 20th century new chemical classes of organic dyes and pigments continued to be discovered. Probably the most significant discovery was that of the phthalocyanines, which have become established as the most important group of blue and green organic pigments. As with virtually every other new type of chromophore developed over the years, the discovery of the phthalocyanines was fortuitous. In 1928, chemists at Scottish Dyes, Grangemouth observed the presence of a blue impurity in certain batches of phthalimide produced from the reaction of phthalic anhydride with ammonia. They were able to isolate the blue impurity and subsequently its structure was established as iron phthalocyanine. The source of the iron proved to be the reactor vessel wall, which had become exposed to the reactants as a result of a damaged glass lining. Following this discovery, the chemistry of formation of phthalocyanines and their chemical structure and properties was investigated extensively by Linstead of Imperial College, London. Copper phthalocyanine (13) emerged as by far the most important product, a blue pigment which is capable of providing a brilliant intense blue colour and excellent technical performance, yet which at the same time can be manufactured at low cost in high yield from commodity starting materials (see Chapter 5). The discovery of this unique product set new standards for subsequent developments in dye and pigment chemistry.
|
|
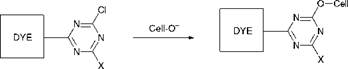
As time progressed, the strategies adopted in dye and pigment research evolved from those used in the early approach which were based largely on empiricism and involved the synthesis and evaluation of large numbers of products, to a more structured approach involving more fundamental studies of chemical principles. For example, attention turned to the reaction mechanisms involved in the synthesis of dyes and pigments and to the interactions between dye molecules and textile fibres. Probably the most notable advance in textile dyeing which has taken place during the 20th century and which, it may be argued, emerged from such investigations is the process of reactive dyeing. Reactive dyes contain functional groups which, after application of the dyes to certain fibres, can be linked covalently to the polymer molecules that make up the fibres, and this gives rise to dyeings with superior washfastness compared with the more traditional dyeing processes. Dyes which contain the 1,3,5-triazinyl group, discovered by ICI in 1954, were the first successful group of fibre-reactive dyes. The introduction of these products to the market as Procion dyes by ICI in 1956, initially for application to cellulosic fibres such as cotton, proved to be a rapid commercial success. The chemistry involved when Procion dyes react with the hydroxyl groups present on cellulosic fibres under alkaline conditions involves the aromatic nucleophilic substitution process outlined in Scheme 1.1, in which the cellulosate anion is the effective nucleophile. Reactive dyes have developed into one of the most important application classes of dyes for cellulosic fibres, and their use has been extended to a certain extent to other types of fibres, notably wool and nylon (see Chapter 8).
|
Procion (1,3,5-triazinyl) reactive dye Covalently-bonded dye |
Scheme 1.1 The reaction of Procion dyes with cellulosic fibres
In the latter part of the 20th century, new types of dyes and pigments for the traditional applications of textiles, leather, plastics, paints and printing inks continued to be developed and introduced commercially but at a declining rate. It is clear that the colour manufacturing industries considered that a mature range of products existed for these conventional applications and elected to transfer research emphasis towards process and product development, and the consolidation of existing product ranges. At the same time, during this period, research effort in organic colour chemistry developed in new directions, sustained by the opportunities presented by the emergence of a range of novel applications demanding new types of colorants. These colorants have commonly been termed ‘functional dyes’ because the applications require the dyes to perform certain functions beyond simply providing colour. The applications of functional dyes include some of the more recently developed reprographic techniques, such as electrophotography and ink-jet printing, a wide range of electronic applications including optical data storage, liquid crystal displays, lasers and solar energy conversion, and a range of medical uses (see Chapter 10). For these new applications, and also for traditional uses, concepts of molecular design of new dyes for specific properties have become increasingly important. Of particular significance in this respect is the fact that quantum mechanical methods have become accessible as a routine tool for the calculation of a range of properties of dyes, facilitated by the rapid advances in computing technology. The Pariser-Pople-Parr (PPP) molecular orbital method has proved of particular value for this purpose, although a range of more sophisticated methods are becoming increasingly accessible. From a knowledge of the molecular structure of a dye, these methods may be used with a reasonable degree of confidence to predict by calculation its colour properties, including the hue of the dye from its calculated absorption maximum, and its intensity as indicated by its molar extinction coefficient. The availability of these methods allows the potential properties of large numbers of molecules to be screened as an aid to the selection of synthetic target molecules (see Chapter 2).
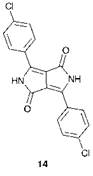
Probably the most important new chromophoric system to be discovered in more recent years is the diketopyrrolopyrroles (DPP), exemplified by compound 14, which are a series of high performance brilliant red pigments that exhibit properties similar to the phthalocyanines. The formation of a DPP molecule was first reported in 1974 as a minor product obtained in low yield from the reaction of benzonitrile with ethyl bromoacetate and zinc. A fascinating study by research chemists at Ciba Geigy into the mechanistic pathways involved in the formation of the molecules led to the development of an efficient ‘one-pot’ synthetic procedure for the manufacture of DPP pigments from readily available starting materials (see Chapter 4). The development of DPP pigments has emerged as an outstanding example of the way in which an application of the fundamental principles of synthetic and mechanistic organic chemistry can lead to an important commercial outcome. There are other
|
|
important lessons for colour chemists in this discovery. It demonstrates that, well over a century after Perkin’s discovery of Mauveine, there still remains scope for the development of new improved colorants for traditional colour applications. In addition, while powerful sophisticated molecular modelling methods are now available to assist in the design of new coloured molecules, the perception to follow-up and exploit the fortuitous discovery of a coloured compound, perhaps as a trace impurity in a reaction, will remain a vital complementary element in the search for new dyes and pigments.
The increasing public sensitivity towards the environment has in recent years had a major impact on the chemical industry. There is no doubt that one of the most important challenges to chemists currently is the requirement to satisfy the increasingly stringent toxicological and environmental constraints placed on industry as a consequence not only of government legislation but also of public perception. Since the products of the colour industry are designed to enhance our living environment, it may be argued that this industry has a special responsibility to ensure that its products and processes do not have an adverse impact on the environment in its wider sense (see Chapter 11).
 18 августа, 2015
18 августа, 2015  Pokraskin
Pokraskin 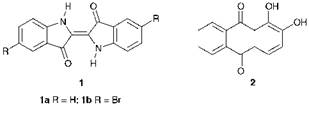
 Опубликовано в рубрике
Опубликовано в рубрике 