Chromium(III) oxide crystallizes in the rhombohedral structure of the corundum type; space group D3d6 -R3c, p = 5.2 g cm-3. Because of its high hardness (ca. 9 on the Mohs scale) the abrasive properties of the pigment must be taken into account in certain applications [3.54]. It melts at 2435 °C but starts to evaporate at 2000 °C. Depending on the manufacturing conditions, the particle sizes of chromium oxide pigments are in the range 0.1-3 pm with mean values of 0.3-0.6 pm. Most of the particles are isometric. Coarser chromium oxides are produced for special applications, e. g., for applications in the refractory area.
Chromium oxide has a refractive index of ca. 2.5. Chromium oxide green pigments have an olive green tint. Lighter greens with yellowish hues are obtained with finely divided pigments, and darker, bluish tints with larger particle diameters; the darker pigments are weaker colorants. The maximum of the reflectance curve lies in the green region of the spectrum at ca. 535 nm (Figure 3.6, curve a). A weaker maximum in the violet region (ca. 410 nm) is caused by Cr-Cr interactions in the crystal lattice. Chromium oxide green pigments are used in IR-reflecting camouflage coatings because of their relatively high reflectance in the near infrared (Figure 3.6, curve b).
|
Fig. 3.6 Dependence of the reflectance of chromium oxide on the wavelength. a) Regular pigment, b) special product with larger particle size and high IR reflectance. |
Since chromium(III) oxide is virtually inert, chromium oxide green pigments are remarkably stable. They are insoluble in water, acid, and alkali and are thus extremely stable to sulfur dioxide and in concrete. They are light-, weather-, and temperature — resistant. A change of the tint only occurs above 1000 °C due to particle growth.
3.1.2.2
 24 октября, 2015
24 октября, 2015  Pokraskin
Pokraskin 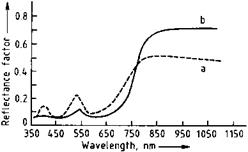
 Опубликовано в рубрике
Опубликовано в рубрике 