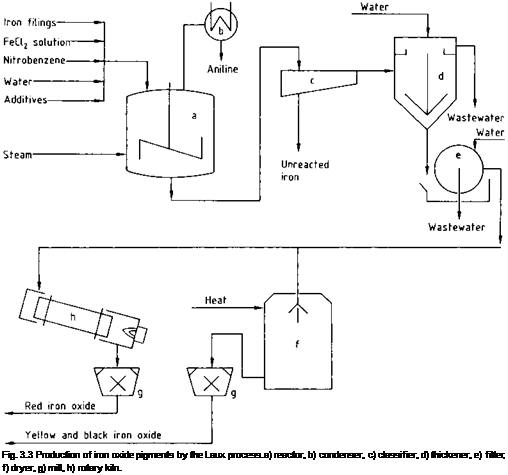
The Bechamp reaction (i. e., the reduction of aromatic nitro compounds with antimony or iron), which has been known since 1854, normally yields a black-gray iron oxide mud that cannot be transformed into an inorganic pigment. By adding iron(II) chloride or aluminum chloride solutions, sulfuric acid, and phosphoric acid, Laux modified the process to yield high-quality iron oxide pigments (Eq. (3.8) and (3.12)) [3.40]. Many types of pigments can be obtained by varying the reaction conditions. The range extends from yellow to brown (mixtures of a-FeOOH and/or a-Fe2O3 and/or Fe3O4) and from red to black. If, for example, iron(II) chloride is added, a black pigment with very high tinting strength is produced [3.40]. However, if the nitro compounds are reduced in the presence of aluminum chloride, high-quality yellow pigments are obtained [3.41]. Addition of phosphoric acid leads to the formation of light to dark brown pigments with good tinting strength [3.42]. Calcination of these products (e. g., in rotary kilns) gives light red to dark violet pigments (Eq. (3.2)). The processes are illustrated in Figure 3.3.
The type and quality of the pigment are determined not only by the nature and concentration of the additives, but also by the reaction rate. The rate depends on the grades of iron used, their particle size, the rates of addition of the iron and nitrobenzene (or other nitro compound), and the pH value. No bases are required to precipitate the iron compounds. Only ca. 3% of the theoretical amount of acid is required to dissolve all of the iron. The aromatic nitro compound oxidizes the Fe[2]+ to Fe3+ ions, acid is liberated during hydrolysis and pigment formation, and more metallic iron is dissolved by the liberated acid to form iron(II) salts; consequently, no additional acid is necessary.
The iron raw materials used are grindings from iron casting or forging that must be virtually free ofoil and grease. The required fineness is obtained by size reduction in edge runner mills and classification with vibratory sieves. The iron and the nitro compound are added gradually via a metering device to a stirred tank (a) containing the other reactants (e. g., iron(II) chloride, aluminum chloride, sulfuric acid, and phosphoric acid). The system rapidly heats up to ca. 100 °C and remains at this temperature for the reaction period. The nitro compound is reduced to form an amine (e. g., aniline from nitrobenzene), which is removed by steam distillation. Unreacted iron is also removed (e. g., in shaking tables, c). The pigment slurry is diluted with water in settling tanks (d) and the pigment is washed to remove salts, and filtered on rotary filters (e). It may then be dried on a band, pneumatic conveyor, or spray dryers to form yellow or black pigments, or calcined in rotary kilns (h) in an oxidizing atmosphere to give red or brown pigments. Calcination in a nonoxidizing atmosphere at 500-700 °C improves the tinting strength [3.43]. The pigments are then ground to the desired fineness in pendular mills, pin mills, or jet mills, depending on their hardness and application.
 |
The Laux process is a very important method for producing iron oxide because of the coproduction of aniline; it does not generate byproducts that harm the environment.
 20 октября, 2015
20 октября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 