In principle, all iron oxide hydroxide phases can be prepared from aqueous solutions of iron salts (Table 3.2). However, precipitation with alkali produces neutral salts (e. g., Na2SO4, NaCl) as byproducts, which enter the wastewater.
Precipitation is especially suitable for producing soft pigments with a pure, bright hue. The manufacture of a-FeOOH yellow is described as an example (Eq. (3.6)). The raw materials are iron(II) sulfate (FeSO4 7 H2O) or liquors from the pickling of iron and steel (FeSO4 or FeCl2 [3.18]), and alkali (e. g. NaOH, Ca(OH)2, ammonia, or magnesite [3.19]). The pickle liquors usually contain appreciable quantities of free acid, and are therefore first optionally neutralized by reaction with scrap iron. Other metallic ions should not be present in large amounts, because they have an adverse effect on the hue of the iron oxide pigments.
The solutions of the iron salts are first mixed with alkali in open reaction vessels (Figure 3.2, left-hand side) and oxidized, usually with air. The quantity of alkali used is such that the pH remains acidic. The reaction time (ca. 10-100 h) depends on the temperature (10-90 °C) and on the desired particle size of the pigment. This method yields yellow pigments (a-FeOOH) [3.20, 3.21]. If yellow nuclei are produced in a separate reaction (Figure 3.2, tank a), highly consistent yellow iron oxide pigments with a pure color can be obtained [3.22].
|
Fig. 3.2 Production of yellow iron oxide by the precipitation (left) and Penniman (right) process. a) seeding tank, b) precipitation pigment reactor, c) Penniman pigment reactor with scrap basket, d) filter, e) dryer, f) mill. |
If precipitation is carried out at ca. 90 °C while air is passed into the mixture at ca. pH > 7, black iron oxide pigments with a magnetite structure and a good tinting strength are obtained when the reaction is stopped at a FeO : Fe2O3 ratio of ca. 1 : 1 (Eq. (3.10)). The process can be accelerated by operating at 150 °C under pressure; this technique also improves pigment quality [3.23]. Rapid heating of a suspension
of iron oxide hydroxide with the necessary quantity of Fe(OH)2 to ca. 90 °C also produces black iron oxide (Eq. (3.11)) of pigment quality [3.24, 3.25].
Orange iron oxide with the lepidocrocite structure (y-FeOOH) is obtained if dilute solutions of the iron(II) salt are precipitated with sodium hydroxide solution or other alkalis until almost neutral. The suspension is then heated for a short period, rapidly cooled, and oxidized (Eq. (3.5)) [3.26, 3.27].
Very soft iron oxide pigments with a pure red color may be obtained by the direct red-method: first preparing a-Fe2O3 nuclei, and then continuously adding solutions of iron(II) salt with atmospheric oxidation at 80 °C. The hydrogen ions liberated by oxidation and hydrolysis are neutralized by adding alkali and keeping the pH constant [3.28]. Pigment-quality a-Fe2O3 is also obtained when solutions of an iron(II) salt, preferably in the presence of small amounts of other cations, are reacted at 60-95 °C with excess sodium hydroxide and oxidized with air [3.29].
The Penniman process is probably the most widely used production method for yellow iron oxide pigments [3.30, 3.31]. This method considerably reduces the quantity of neutral salts formed as byproducts. The raw materials are iron(II) sulfate, sodium hydroxide solution, and scrap iron. If the sulfate contains appreciable quantities of salt impurities, these must be removed by partial precipitation. The iron must be free of alloying components. The process usually consists of two stages (Figure 3.2, right-hand side).
In the first stage, nuclei are prepared by precipitating iron(II) sulfate with alkali (e. g., sodium hydroxide solution) at 20-50 °C with aeration (tank a). Depending on the conditions, yellow, orange, or red nuclei may be obtained. The suspension of nuclei is pumped into vessels charged with scrap iron (reactor c) and diluted with water. Here, the process is completed by growing the iron oxide hydroxide or oxide onto the nuclei. The residual iron(II) sulfate in the nuclei suspension is oxidized to iron(III) sulfate by blasting with air at 75-90 °C. The iron(III) sulfate is then hydrolyzed to form FeOOH or a-Fe2O3. The liberated sulfuric acid reacts with the scrap iron to form iron(II) sulfate, which is also oxidized with air (Eq. (3.7 a-c)). The reaction time can vary from days to several weeks, depending on the conditions chosen and the desired pigment. At the end of the reaction, metallic impurities and coarse particles are removed from the solid with sieves or hydrocyclones; water-soluble salts are removed by washing. Drying is carried out with band or spray dryers (e) and disintegrators or jet mills are used for grinding (f). The main advantage of this process over the precipitation process lies in the small quantity of alkali and iron(II) sulfate required. The bases are only used to form the nuclei and the relatively small amount of iron(II) sulfate required initially is continually renewed by dissolving the iron by reaction with the sulfuric acid liberated by hydrolysis. The process is thus considered environmentally friendly. The iron oxide pigments produced by the Penniman process are soft, have good wetting properties, and a very low flocculation tendency [3.30-3.38].
Under suitable conditions the Penniman process can also be used to produce reds directly. The residual scrap iron and coarse particles are removed from the pigment, which is then dried [3.39] and ground using disintegrators or jet mills. These pigments have unsurpassed softness. Depending on the raw materials, they usually have purer color than the harder red pigments produced by calcination, but
the moisture content is higher and the change in hue with intensive milling should not be neglected.
 19 октября, 2015
19 октября, 2015  Pokraskin
Pokraskin 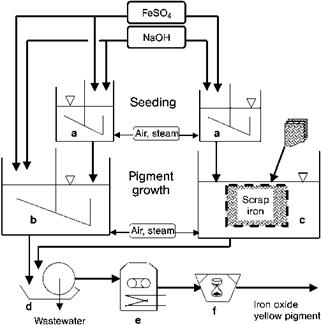
 Опубликовано в рубрике
Опубликовано в рубрике 