During the early 1940s, the first aluminum components were bonded by means of a hot-setting phenolic resin plastified with polyvinyl formal, called ‘Redux’ (see Chapter 2 and Section 5.4). High-strength and fatigue-resisting joints were obtained when aluminum alloys were pickled with an aqueous solution of sulfuric acid and sodium dichromate at 60 ° C for 30 min before application of the adhesive. This so — called ‘pickling process’ was later used worldwide. Redux became history, and still today, it is sometimes used (almost unchanged) in aircraft construction.
Redux was a two-part adhesive consisting of a liquid phase of phenolic resin applied onto the metals to be bonded, which in turn were coated with a primer made from a similar type of material. Polyvinyl formal, which is solid (granular) at room temperature, was then sprinkled onto the metal adherents; any polyvinyl formal residues that did not adhere to the surface of the phenolic resin were then blown off using compressed air. By using this somewhat original procedure, the adhesive components were reliably and satisfactorily mixed and dosed. Following their application to both sides, the parts to be bonded were joined together, subjected to a pressure of up to approximately 8 bar (8 x 105 N m~2), and then heated for 30-45 min after a temperature of 170 °C had been reached. The granular polyvinyl formal (i. e. the thermoplastic component) melted, and the phenolic system cured in
the form of a polycondensation. The water formed during the reaction was expelled from the bond-line due to the high pressure applied. About 75% of the adhesive layer was composed of polyvinyl formal and 25% of cured phenolic resin, in an inhomogeneous distribution. Such a distribution was found to be advantageous with regard to fracture mechanics, and a high polyvinyl content was required to obtain adequate peel strength of the joint, although the latter had a relatively low resistance to heat up to a temperature of approximately 60-70 °C [2].
The features of the bonded structures were so compelling that bonding technology was used in the ‘Dove’, an early commercial aircraft manufactured by De Havilland and, on a larger scale, in the first heavy commercial jet aircraft of the world, the ‘Comet’, which was produced by the same manufacturer [3]. Particularly in the fuselage of the Comet, both bonded stringers and doublers in the area of the windows were employed. Unfortunately, some of these four-engined aircraft suffered disastrous crashes due to structural failure. Originally, it had been assumed that the bonded joints might have been the reason for these catastrophes, but no evidence was found for this assumption. On the contrary, it is well known that the bonded joints used in the Comet, and later also in the ‘Trident’, did not present any problems and were in an absolutely perfect condition after a service life of 10-15 years.
In the twin-engine Turboprop Type Fokker 27 ‘Friendship’, which entered service in 1953-1954, almost 70% of the total structure (nearly 550 components) was bonded with phenolic resin. This has been, to this day, the most consistent application of bonding technology in aircraft, both in the fuselage and in the airfoil structures. In total, 1000 of these aircraft were manufactured, with some operating for up to 30 years. The more up-to-date version of the Fokker F 50, with a similar configuration, is a reliable ‘workhorse’ for short-distance flights worldwide. The twin-engined jet airplane Fokker F 34 and its successor, the Fokker F 100, both of which were manufactured later, incorporated structures that were mostly bonded with phenolic resin and which proved satisfactory for a long time owing to the extremely good fatigue properties and excellent aerodynamic smoothness of the outer structures. Damage to the adhesive joints never occurred, owing largely to the fact that during construction of the Comet and later of the Fokker aircraft, anodization with chromic acid was used. This approach, following chromosulfuric acid surface preparation, made it possible to create relatively thick porous oxide structures on the aluminum which had good corrosion properties owing to their fully developed barrier layer (see Chapter 3 and Section 7.5). Originally, this anodization process was not introduced to optimize the bonding process, but rather to optimize the corrosion characteristics of the nonbonded area of the aluminum surface that had also been coated with a phenolic resin primer and cured separately before the adhesive was applied. It was assumed at that time that the water liberated during the setting of the primer, in the presence of phenolic resin molecules, contributed to a sealing effect of the aluminum oxides, thereby ‘clamping’ the primer within the rearranging aluminum oxide structures. Experience then taught that anodization did not affect the properties of the bonds, and consequently this manufacturing process was adhered to, without realizing that this combination of surface preparation, adherent and adhesive was near-perfect with regards to a resistant adhesion (see Chapter 3).
The bonding of high-strength aluminum alloys using phenolic resins was a worldwide success (which today can be explained scientifically), even in aircraft structures with long-term use. Little attention was paid to the problems of aging resistance of bonded metallic structures in humid conditions with possibly corrosive attacks, especially in aircraft manufacture. However, during the 1960s, in aircraft manufacture, the relatively brittle phenolic resins with low resistance to peel were gradually replaced by epoxy-based adhesives modified with nitrile rubber that also had a higher resistance to heat up to 80 °C. A formulation developed later required a curing temperature of only 120 °C instead of 170 °C, so that the heating process did not alter the fatigue characteristics of the aluminum alloy. Previously, when using phenolic resin adhesives this alteration was compensated by an appropriate dimensioning of the components. Adhesive joints bonded with epoxy resins were shown partly to have a higher strength and fatigue resistance than phenolic resins. The results of aging tests performed in humid and corrosive environments showed that anodizing had no better effect than merely pickling in chromosulfuric acid. Today, these tests are considered to have been completely inadequate, with little attention having been paid to the fracture surface analysis ofthe destroyed specimen following the aging processes. Nonetheless, anodizing was abandoned in some parts of the world in order to save costs. When long-term damage occurred in the form of a delamination near the interface, as well as corrosion penetrating the bond-line after a service life of about two years, anticorrosive primers based on epoxy and phenolic components were developed, since experience had taught us that phenolic resins were very efficient. Hence, strontium chromate was added as anticorrosive agent, especially for the case of damage. Although, especially in the United States and during manufacture of the Airbus, chromosulfuric acid was used for surface preparation, Fokker and the British aircraft manufacturers never abandoned the use of conventional adhesive systems with anodization [4, 5].
The decision to abandon anodization for adhesive systems based on a combination of epoxy/phenolic primers and epoxy nitrile adhesives proved to be wrong. As the author remembers, this decision was rather taken by intuition, since the interaction between phenolic and epoxy resins and aluminum oxides (which was an important prerequisite for resistant adhesion) was not yet well known, and hence neither was the importance of anodization recognized. Damage of these bonded joints occurred worldwide in the form of delaminations and bond-line corrosion after operation periods of two to five years in military aircraft (first during the War in Vietnam) and later also in civil aircraft (see Section 7.6). The delaminations reported by Boeing as early as the mid-1970s were found to be adhesion failure between the primers and the metal surfaces (Figure 8.4), partly in the form of corrosion of the adherents (which had rapidly invaded the bond-line from the poorly protected edges), and also in the form of delaminations between the primer and adherents occurring in front of the corrosion areas.
At that time, basic research into the long-term behavior of adhesion was started worldwide, with especially important contributions being made in Germany [6]. It was discovered that, in epoxy resin adhesive systems cured with dicyandiamide, a high alkalinity developed as soon as the system was attacked by water. This was due to
|
Figure 8.4 Part of a delaminated window doubler of a civil aircraft. The visible corrosion traces are caused by humidity invading after delamination. |
the presence of rests of reactive dicyandiamide and reaction products of dicyandia — mide and aluminum oxides that induced the liberation of amines. In the presence of the polymer, the aluminum oxides were destabilized by this alkalinity, such that an accelerated solvolysis was initiated that induced macroscopic damages on the surface. By nature, these damages occurred more rapidly on surfaces that were only pickled and where the oxide layers were only about 30 nm thick, than on anodized surfaces where the layer thickness was up to 2 pm. In the latter case, destruction of the bond by solvolysis would only occur after 40-50 years of operation, as may be estimated today. It is known that, at a pH greater than 7 (i. e. in the alkaline range), aluminum oxides are not stable with regards to solvolysis, but they are highly stable at acid pH (5.5-6.5). This explains the fact that no such damages occurred in phenolic resin bonds since, even in the cured condition, phenolic resins continue to remain slightly acid when water penetrates into the bond. The results are a stabilization of the oxides with regards to solvolysis. Phenolic resins may also form water-resistant chelate compounds with aluminum oxides (see Chapter 3). Whilst there is no doubt that chelate compounds may also be formed with phenolic epoxy resin primers, the remaining alkalinity of the overall adhesive layer may penetrate these primers in the case of an extremely humid environment and begin to destabilize the oxide layer.
As a consequence of these research investigations, anodization was reintroduced into aircraft manufacture worldwide, with Boeing, in the United States, applying phosphorus anodization, and the European aircraft manufacturers carrying out anodization with chromic acid. Today, with careful working procedures, high — performance adhesive qualities can be achieved when using either method, in combination with anticorrosive primers and epoxy resin adhesives, provided that the edges are protected both structurally and chemically against primary corrosion. In this way a structural service life of more than 30 years is easily obtained for commercial aircraft. At present, anodization with chromic acid is steadily being replaced by alternative anodization methods.
The development ofbonding technology in aircraft manufacture was considerably impaired by the above-described problems, particularly with regards to metal bonding. However, this does not apply to the same extent to fiber composites, which will surely increasingly replace metal structures in large civil and military aircraft (as will be illustrated later), and which may be easily bonded.
 5 ноября, 2015
5 ноября, 2015  Pokraskin
Pokraskin 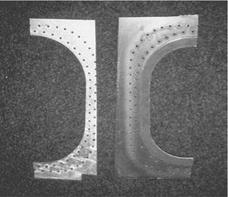
 Опубликовано в рубрике
Опубликовано в рубрике 