To date, it has not been possible to create durable adhesive bonds with aluminum alloys using structural adhesives unless a special surface preparation is performed prior to bonding. This will be illustrated on EN-AW (AlCuMg) and EN-AW 5182 (AlMg4.5Mn0.4), which are noncurable wrought alloys. It is therefore of great interest to identify the surface conditions and surface preparation methods, respectively, which provide for good durability. An overview is provided in Table 7.2 of the surface preparations used for the above-mentioned aluminum alloys bonded with a one-part, hot-setting epoxy resin adhesive manufactured by Dow Automotive.
When used for strength tests of structural adhesive bonds, single lap-shear specimens produced according to DIN 1465 have well-known disadvantages. However, this simple sample geometry was employed here because a great number of parameters were tested. It seems as if these specimens are perfectly suitable and still widely used in practice to compare the aging characteristics of adhesives as a function of surface preparation and aging simulation. Following the aging process, the loss in strength is related to the initial strength, and the failure patterns are analyzed. Only in few cases were the so-called ‘tensile shear specimens with thick adherents’ used for the investigation of the shear stress-shear deformation behavior.
The durability tests were performed with the adhesives, substrates, and aging simulations that are mainly used in the automobile industry (Tables 7.3 and 7.4). The artificial aging procedures were divided roughly into categories according to the major failure mechanism. For example, in the salt spray test and the VDA 621-415 or VDA KKT test, failure is induced by corrosion, whereas in the climate change test and the immersion test, it is mainly induced by humidity or water.
For representation of the test results, conventional bar diagrams were hardly suited due to the great number of parameters investigated. Moreover, the test focused on the influence of the surface preparation and the aging simulation on the residual strength of the assemblies rather than on absolute strength values.
Therefore, the results were represented graphically so as to allow the percentaged residual strength after aging to be visualized, and to make it possible to differentiate between the major failure mechanisms occurring with different aging simulations — that is, ‘humidity’ or ‘salt’ (= corrosion induced by exposure to chloride ions).
|
Table 7.2 Surface preparation methods for aluminum alloys.
|
It becomes obvious that aging in corrosive media caused a relatively important loss in strength if the adherent surfaces were vapor-degreased with acetone (Figure 7.36) or blasted with corundum (Figure 7.37) prior to bonding. The failure mechanism that started from the adherent edges and progressed towards the inner region of the bonding (bond-line corrosion; Figure 7.38) can easily be detected by visual analysis of the fracture surfaces. In salt-free aging simulations with exposure to humidity, a relatively good durability was achieved when the adherent surfaces were grit-blasted with corundum prior to bonding. In all tensile lap-shear specimens there was cohesive failure (near the interface).
If the surfaces of aluminum adherents are pretreated with a mild alkaline (e. g. sodium metasilicate containing solution; ‘Bonder’) prior to bonding, the bonded joints have a near-opposite aging behavior. The surface preparation generates a silicate coating (conversion coat) that clearly protects the metal against corrosive attack by chloride ions. In the presence of humidity, however, the conversion coat
|
Table 7.3 Aging simulations with failure mainly induced by corrosion.
Note: After removal from the aging simulation, all samples were conditioned at 40 °C for three days, and tested at room temperature. |
seems to induce local alkalization of the damage medium within the interface (Figure 7.39).
Following artificial aging in standardized corrosion tests (e. g. salt spray test, VDA alternating climate test 621-415), there was only a relatively low percentage loss in strength. After four weeks of storage in a water bath at 70 ° C, however, the diffusion of humidity into the interface, accelerated by the addition of a tenside, induced a dramatic loss in strength and correlated well with the results obtained following aging under natural climatic conditions. Neither of the other short-term aging tests (VW P1200, cataplasm test, etc.) with mainly humidity-induced failure mechanisms was able to simulate this damage pattern, with the exception of the three-months VDA KKT test which combined alternating exposure to humidity and temperature with corrosive exposure similar to outdoor weathering. There was also a significant loss in strength, similar to the above-mentioned damage pattern.
As indicated by the fracture surfaces (Figure 7.40), the adhesive was almost completely delaminated; this can be explained by a degradation of the aluminum oxide layer that is only stable in a pH range between 4.3 and 8.5.
The aging simulation tests were then performed with bonded joints that had undergone a chromate-free, no-rinse TiZrO conversion surface preparation. No
|
Table 7.4 Aging simulations with failure mainly induced by humidity.
Note: After removal from the aging simulation, all samples were conditioned at 40 °C for three days, and tested at room temperature. |
significant increase in durability was obtained compared to merely acetone-degreased reference bondings (Figure 7.41).
The aluminum alloy used here (AlMg4.5Mn0.4) was comparable to the AlMg3 substrates with regard to its technical and electrochemical characteristics according to the manufacturer’s instructions.
Figure 7.42 shows the results obtained with a tenside-based dry lubricant coat applied as temporary anticorrosive agent and forming aid as used in pressing lines. Apparently, this was not sufficiently absorbed by the polymer, and caused an impaired adhesion. All aging simulations showed in common a rather important loss in
|
Residual strength (%) following aging related to the initial strength (19,8 N mm Adhesive: One-part ЕР BM XD 4600 Substrate: Al Mg 3 |
-2) |
salt |
|
salt spray (1000 h) |
||
|
natural weathering (365 d) |
^,100′ |
^salt spray (2000 h) |
|
water immersion 70 °С + detergent (28 d) (С// |
yeo 40 |
VDA 621-415 (10 cycles) |
|
20 |
||
|
condensation water 40 °С (28 d) |
/Vtightend natural weathering (183 d) |
|
|
cataplasma humide (28 d) ^ |
P 1200 (60 cycles) |
|
|
, ж VW P 1200 (120 cycles) moisture □ Degreased in acetone |
|
Figure 7.36 Aging characteristics of bonded aluminum after acetone degreasing. |
|
Residual strength (%) following aging related to the initial strength (19,7 N mm-2) Adhesive: One-part ЕР BM XD 4600 Substrate: Al Mg 3 |
salt |
|
salt spray (1000 h) |
|
|
natural weathering (365 d) /V^80 |
^^^^salt spray (2000 h) |
|
water immersion 70 °С + detergent (28 d) с/ ^ 40 |
621-415 (10 cycles) |
|
m Ns^° |
|
|
condensation water 40 °С (28 d) \ / |
‘/ / / tightend natural weathering (183 d) |
|
cataplasma humide (28 d)N^’—^^j |
A/V P 1200 (60 cycles) |
|
VW P 1200 (120 cycles) moisture □ Grit-blasted (corundum) |
|
Figure 7.37 Aging characteristics of bonded aluminum after grit-blasting (corundum). |
strength (Figure 7.42). It should be noted, however, that some aspects relevant to the manufacture processes used in the automobile industry were disregarded. For example, the dry lubricant coat beneath the noncrosslinked polymer must be resistant to being washed away during the washer processes prior to e-coating.
Specific tests on steel bondings are already in progress. Although representative only to a limited extent, the results obtained with the drylub-coated aluminum substrate used in these tests correlated well with the results of investigations
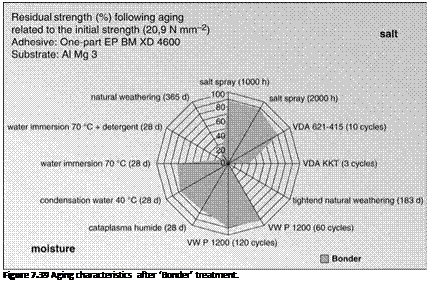 |
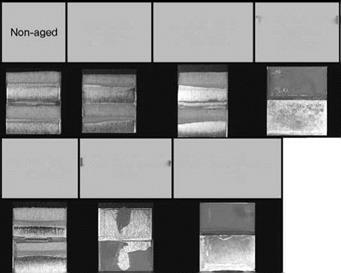
 Figure 7.38 Fracture surfaces after grit-blasting.
Figure 7.38 Fracture surfaces after grit-blasting.
performed by Kleinert et al., who studied, among others, parameters that were comparable to those tested here [18].
The aging characteristics were most favorable following alkaline or acid etching. The chemical removal of contaminated covering layers by means of a sodium hydroxide solution (Figure 7.43) is an established etching method for aluminum, whereas the surface preparation with high-percentage nitric acid (Figure 7.44) is rather unconventional. Concentrated nitric acid barely attacks aluminum, and consequently there is no removal of the covering layers by the etching process in the conventional sense. In principle, this method is only suitable for closed systems, as nitrogen gases may develop. Following both surface preparations, the bonded joints showed a very good resistance to salt-containing aging media. Under exposure to humidity, their durability also was at least comparable to that of other surface preparations (Figures 7.43 and 7.44). Both, before and after aging (performed in accordance with EN ISO 10365), all fracture surfaces had a particular cohesive failure pattern in proximity to the substrate (SCF).
|
Salt spray |
VDA 621-415 |
|
(2000 h) |
(10 cycles) |
|
Tightened natural weathering (183 d) |
![]()
|
Figure 7.40 Fracture surfaces after ‘Bonder’ treatment.
Figure 7.41 Aging characteristics after TiZrO conversion treatment. |
It has become obvious that, when it comes to evaluating the durability of epoxyresin bonded aluminum following different surface preparations, the significance of standardized aging tests may not be satisfactory. The influence of the simulated aging processes on the technical characteristics of bonded aluminum depends both on the type, duration and intensity of the major failure mechanism, and on the substrate surface conditions generated by the specific surface preparation method. Hence, the
|
Figure 7.42 Aging characteristics after TiZrO + Drylub. |
|
Residual strength (%) following aging related to the initial strength (21,3 N mm-2) Adhesive: One-part ЕР BM XD 4600 Substrate: Al Mg 3 |
salt |
|
salt spray (1000 h) |
|
|
natural weathering (365 d) |
Tii^y-^salt spray (2000 h) |
|
water immersion 70 °С + detergent (28 d) ^ ///9C 40 |
/ \vDA 621*415 (10 cycles) |
|
I /м^^° |
|
|
condensation water 40 °С (28 d) V\ / |
/у tightend natural weathering (183 d) |
|
cataplasma humide (28 d) |
P 1200 (60 cycles) |
|
, . VW P 1200 (120 cycles) moisture □ Alkaline etching in NaOH |
|
Figure 7.43 Aging characteristics after alkaline etching in NaOH. |
surface preparation has a major effect on the sensitivity of the bonding to different aging processes.
The intensification of the individual parameters evaluated in short-term tests (e. g. temperature, humidity, concentration of chloride ions) does not necessarily induce the desired acceleration effect. Therefore, a correlation with natural aging processes is possible only to a very limited extent.
|
Residual strength (%) following aging related to the initial strength (20,7 N mm Adhesive: One-part ЕР BM XD 4600 Substrate: Al Mg 3 |
-2) |
salt |
|
salt spray (1000 h) |
||
|
natural weathering (365 d) |
_100- |
""—salt spray (2000 h) |
|
—"80 |
||
|
water immersion 70 °С + detergent (28 d) |
60 40 |
/ vDA 621-415 (10 cycles) |
|
20 |
||
|
condensation water 40 °С (28 d) |
Ту tightend natural weathering (183 d) |
|
|
cataplasma humide (28 d) ^ |
P 1200 (60 cycles) |
|
|
, . VW P 1200 (120 cycles) moisture □ Acid etching in cone, nitric acid |
|
Figure 7.44 Aging characteristics after acid etching in concentrated nitric acid. |
In order to assess satisfactorily the durability of epoxy-resin-bonded aluminum, at least in a primary manner, as wide a spectrum of aging simulations as possible is required that must be as close to reality as possible. For evaluating the aging behavior ofbonded aluminum following different surface preparations, the aging simulations were suitably classified according to the major failure-inducing mechanism, such as exposure to humidity or corrosion, although in some aging simulations both mechanisms contribute to failure.
When exposed to corrosive media, bonded aluminum was significantly less durable following mere degreasing or grit-blasting with corundum compared to a chemical surface preparation (see Figures 7.36 to 7.38).
To further investigate this effect, so-called ‘tensile shear specimens with thick adherents’ (DIN 54 451) of a curable wrought aluminum alloy (EN-AW 2017) were subjected to a chemical or mechanical surface preparation and then bonded with a hot-setting, one-part epoxy resin adhesive. Once again, the standardized VDA alternating climate test 621-415 known from the automobile industry was used as aging simulation.
In service, the loads applied can considerably impair the durability of structural polymer-metal joints [16]. Hence, during the artificial aging simulation, some samples were subjected to a static load corresponding to 10% of the initial strength of the bonding, by means of special clamping devices. The shear deformation of the bondings was determined during the test by means of fine-strain extensometers supported close to the lap (resolution: 15 pm).
The t-g diagrams of samples aged under static load compared to non-aged reference samples and reference samples aged without load are shown in Figures 7.45 to 7.48. Likewise, in this test run there was a distinct loss in strength
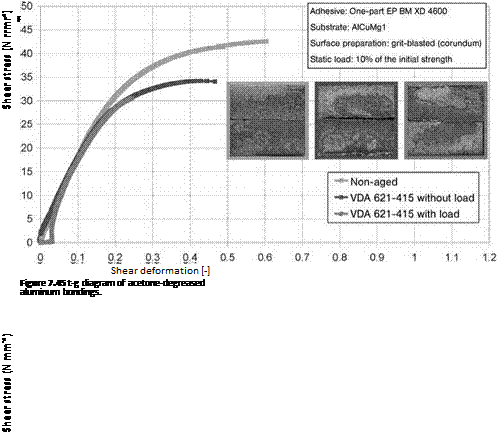
in bondings with degreased or grit-blasted (corundum) adherent surfaces following aging in corrosive media. When a static load was applied, this effect was further enhanced. It was noted that the shear modulus — that is, the quotient from shear stress and shear deformation — decreased in the case of vapor acetone degreasing.
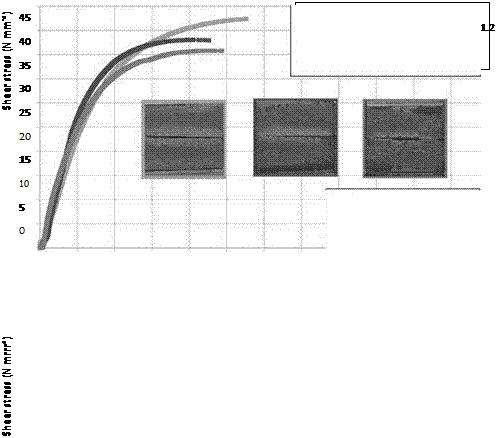
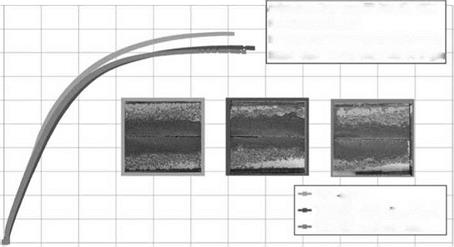 Following 10 cycles of the VDA alternating climate test, a relatively minor loss in strength was detected when the adherent surfaces of bonded aluminum were prepared by alkaline or acid etching prior to bonding (Figures 7.47 and 7.48). The load additionally applied during the aging process did not induce any significant, technically relevant decrease in the shear modulus.
Following 10 cycles of the VDA alternating climate test, a relatively minor loss in strength was detected when the adherent surfaces of bonded aluminum were prepared by alkaline or acid etching prior to bonding (Figures 7.47 and 7.48). The load additionally applied during the aging process did not induce any significant, technically relevant decrease in the shear modulus.
Furthermore, visual inspection of the fracture surfaces revealed that the failure pattern was in analogy to that of bonded, noncurable AlMg3 alloys where bond-line corrosion started from the unprotected edges and progressed towards the inner region of the bonding following acetone degreasing and grit-blasting (corundum) prior to bonding. Following alkaline or acid etching, there was cohesive failure (near the interface).
It is known that surface preparation can have a major influence on the electrochemical potential of metal surfaces. Mechanical surface preparations (e. g. blasting with white corundum) induce residual compressive stresses of around -70 MPa in the AlMg3 surfaces. By cold working, the metallurgical structure becomes susceptible to corrosion that usually starts at the edge and screw dislocations, respectively.
The depth profile of surfaces that were subjected to different surface preparations can be analyzed using surface analysis methods such as secondary neutral particle mass spectrometry (SNMS). In the case of vapor-degreased AlMg3 adherents, an accumulation of magnesium (an alloy component) is detected near the surface.
From the literature it is known that magnesium diffuses from the bulk along the grain boundaries and accumulates in the covering layer of aluminum, which can be explained by the metal’s electrochemical nature. These heterogeneities can impair the resistance of the oxide layer to corrosion and consequently impair the durability of bonded joints [19, 20].
If, in contrast, the naturally grown covering layer is removed by alkaline etching with a sodium hydroxide solution, then no magnesium is detected by means of surface analysis following the generation of these fresh oxide layers.
Following acid etching, the samples have almost identical SNMS depth profiles. Concentrated nitric acid has the interesting property that it only slightly attacks aluminum oxide, despite its usual strong oxidizing effect; consequently, there is no removal of covering layers by etching in the conventional sense. In an acid environment, the magnesium (oxide) phases accumulated at the surface become unstable and are dissolved. In this case, too, the percentage of magnesium detected on the surfaces remains largely below that of the alloy.
At first, it might seem obvious to suspect the electrochemical properties of the adherent surfaces, which are changed by the surface preparations, as being the reason for the dissimilar aging behavior in corrosive media.
Based on this approach, the failure mechanisms detected on the fracture surfaces of the bondings are expected to correlate with electrochemical analyses performed with the adherents following different surface preparations. However, potentiody — namic current density-potential measurements of the aluminum surfaces discussed here do not conform to this hypothesis.
Only in the case of substrates etched with nitric acid is the breakdown potential shifted towards lower potentials. Likewise, only in this case does the passivity with regard to pitting represent an explanatory approach for the good durability of bonded joints exposed to corrosive media following alkaline etching performed prior to bonding.
It was noted that, in the case of etching with concentrated nitric acid, the bondings had a very good durability following aging in corrosive media, despite the rather
|
Figure 7.49 X-ray photoelectron spectroscopy (XPS) analyses of aluminum surfaces prepared with different methods. |
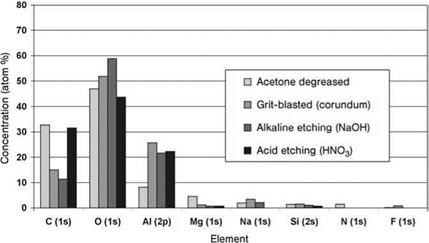
moderate resistance of their surfaces to corrosion. This shows that the attempt to explain exclusively the aging phenomena of bonded joints by the electrochemical properties of the metal surfaces can sometimes be misleading because ‘a priori this approach disregards the adhesion between polymer and substrate as a factor that significantly influences the aging process. The adhesional properties of a metal surface or its oxide layer are significantly determined by its chemical reactivity, its mechanical stability and, moreover, by its morphological characteristics. When the presence of carbon is taken as a hint on organic contamination, X-ray photoelectron spectroscopy (XPS) investigations show that both the abrasive effect of grit-blasting with corundum and chemical removal of covering layers by alkaline etching generate a ‘clean’ (i. e. freshly produced) and therefore reactive surface. In contrast to these surface preparation methods, neither acetone nor concentrated nitric acid can remove the organic contaminations of the aluminum covering layer (Figure 7.49).
As illustrated above, following mechanical surface preparation, aging takes place faster than in the case of samples which were etched with HNO3 and had a good durability. Clearly, the cleaning rate alone, which is achieved by means of a specific surface preparation method, is not pertinent to the assessment and prognosis of the durability of epoxy-resin-bonded aluminum exposed to a corrosive environment.
In this context, a potential effect of the surface morphology generated by the surface preparation is subject to controversy. It has already been shown that the macroscopic dimension (i. e. the surface roughness) classified by several statistical engineering parameters, does not have any major influence. This has been demonstrated, amongst others, both on textured steel sheets (see Section 7.7.7) and on aluminum surfaces pretreated with different surface preparation methods [17]. There appears to be a more significant effect on a submicroscopic level in the dimension of the prepolymers applied. This effect has been extensively investigated on anodized aluminum surfaces [15].
|
Figure 7.50 Model and real structure of an aluminum surface anodized with phosphoric acid [15]. |
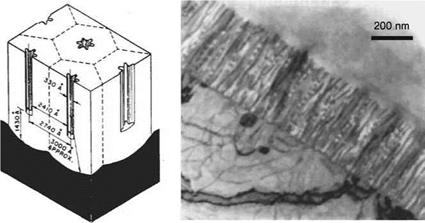
Particularly in the aircraft industry, aluminum materials are subject to an excessively elaborate, multistage anodization procedure in order to optimally prepare their surfaces for bonding. The oxide coatings generated consist of a very thin, almost poreless, dielectric barrier layer, and a partly very fine-pored covering outer layer (Figure 7.50).
For example, with regards to bonding, it is beyond controversy that the anodization of aluminum surfaces with phosphoric acid generates the most favorable aging characteristics compared to other surface preparations. This is explained by a surface enlargement and improved electrochemical stability of the thick oxide layer generated.
Another explanatory approach is to assume that there is a micromechanical interlocking of the polymer after penetration of low-molecular-weight prepolymers into the structures ofthe porous oxide layer. This is quite conceivable when making a rough estimate of the dimensions in question. The diameter of an oxide layer pore generated by anodization ranges from 300 to 500 A, according to the anodization procedure used. An epoxy resin molecule requires an average space of 138 A2, independent of the spatial arrangement of the molecule. The question remains as to how the low mechanical stability of the structures of the oxide coatings generated by anodization with phosphoric acid may contribute significantly to the strength of the adhesive joint system, operating on the premise that there is mechanical adhesion. Moreover, on the basis of this molecular approach, a clearly defined differentiation between chemical effects on the one hand and purely mechanical effects on the other hand seems to be a problem.
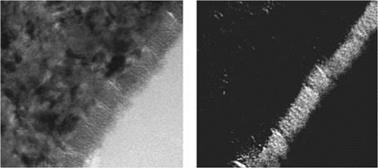
The effect of these different surface preparation methods on the morphology of the solid surface was investigated using transmission electron microscopy (TEM; Figure 7.51). By using ultrathin sections of acetone-degreased or grit-blasted (corundum) aluminum surfaces, a sharp transition with a defined interface can be detected between substrate and polymer, whereas the samples subjected to alkaline and acid
 |
Figure 7.51
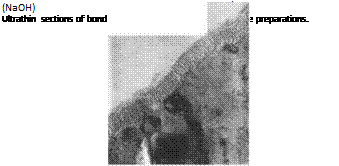 |
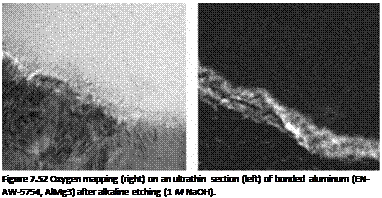
etching had a nonuniform, thin, filigrane structure in this area which turned out to be aluminum oxide, as can be shown by oxide mapping (Figures 7.52 and 7.53).
 |
Once again, the submicroscopic structures of the aluminum surface, together with their good aging characteristics in bonded joint systems exposed to corrosive environments, show that the chemical bonding and debonding reactions do not sufficiently explain the aging processes of metal-polymer joints. The displacement of
|
Figure 7.53 Oxygen mapping (right) on an ultrathin section (left) of bonded aluminum (EN-AW-5754, AlMg3) after acid etching (65% HNO3 solution for 15 min). |
the metal-polymer interface that moves into the nanomorphology of the surface generates an organic/inorganic interphase that is similar to a composite material. This seems to strongly inhibit the kinetics of the attacking failure mechanisms. Furthermore, a more homogeneous transition between solid surface and adhesive is created which may influence both the polymer network configuration and the fracture mechanics of the whole assembly in this area. Finally, it remains possible that the fine-structured oxides, which are found near the interface formed with the adhesive, modify the kinetics and the degree of crosslinking, respectively, inducing an increased molecular mobility and enabling the polymer to build up dynamic adhesion (as described in Chapter 3) which in turn may contribute to generate resistance to water.
Against the background of the problems associated with the aging of metal — polymer assemblies, it seems worthwhile — from an engineering point of view — to develop alternative methods to create such ‘nanomorphologies’ on the adherent surfaces and to take advantage oftheir obviously high effectiveness for the durability of bonded aluminum. The first attempts to replace elaborate etching and anodization procedures with a laser technology-based surface preparation of aluminum have shown great promise [21, 22].
To conclude this discussion it should be noted that the above-described results have been obtained with one-part, hot-setting epoxy resin adhesive-bonded joints. While similar qualitative results can be obtained with bondings using similar adhesives, they generally cannot be found with bondings using adhesives with different chemical formulations. This point is demonstrated in the next section, for bonded steel.
7.7.7
 30 октября, 2015
30 октября, 2015  Pokraskin
Pokraskin 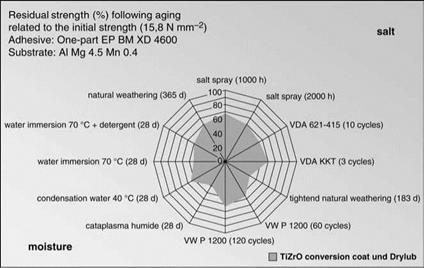
 Опубликовано в рубрике
Опубликовано в рубрике 