In the case of cyanoacrylate adhesives, which are known as ‘Super glues’, curing is initiated by the moisture adsorbed onto the adherents and takes place within the bond-line within a few seconds or minutes. Although cyanoacrylates are easy to process, they cannot overcome gaps which have a width in excess of 0.1 mm. These materials are thermoplastics after curing, and cannot (or can only barely) be plastified; hence, they are mainly suited to the joining of small rigid parts. Some elastomers can also be bonded easily by cyanoacrylate adhesives, for example in the production of packing rings.
The structure of the basic monomer, as well as the anionic polymerization of cyanoacrylate adhesives, is shown in Figure 5.34. The double bond, due to its proximity to two electron-withdrawing groups (the nitrile and the ester group), is extremely sensitive to nucleophilic attack. Such susceptibility provides a monomer the polymerization ofwhich can be initiated by species that are as weakly nucleophilic as water. Various bases can also be used to induce the polymerization of cyanoacrylates, although acids inhibit the process. The extreme reactivity of the monomer results in a fast cure. Cyanoacrylate monomers may contain ester groups from methyl, ethyl to isobutyl, and ethoxy ethyl; acrylates or methacrylates, which are soluble in cyanoacrylate monomers and present a high molecular mass, are frequently added to the basic monomers to increase the viscosity.
It is important to note that cyanoacrylate adhesives are thermoplastics after cure, as this makes them susceptible to creep as well as to attack by moisture. In order to overcome these problems the formulations are sometimes modified by means of crosslinking agents or thermally stable polymers.
|
Figure 5.34 Structure and anionic polymerization of cyanoacrylates. |
Cyanoacrylates were first developed in the United States in 1957 and marketed under the trademark ‘Eastman 90’. Three years later, Sichel introduced them in Germany under the trademark ‘Sicomet’, which now belongs to the Henkel Group. Cyanoacrylate adhesives are used for a wide variety of applications, particularly for the industrial bonding of plastic and rubber materials. They are also employed as tissue adhesives in medicine, or as ‘Super glues’ in home applications.
 23 сентября, 2015
23 сентября, 2015  Pokraskin
Pokraskin 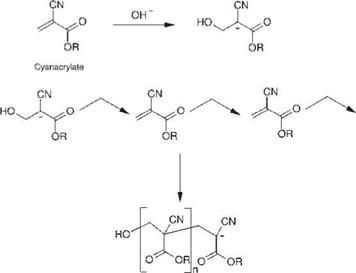
 Опубликовано в рубрике
Опубликовано в рубрике 