Anaerobic adhesives have been available commercially since 1953, the first anaerobic adhesive based on dimethacrylate having been developed that year by Vernon Krieble, in the United States. This was commercialized under the name Loctite [40] and, indeed, the early success of the Loctite Company — which today is the leading manufacturer of acrylate adhesives — is owed to the use of acrylates in adhesive bonding technology.
‘Anaerobic’ is a designation borrowed from biology which signifies that the adhesive remains liquid while it is in contact with the air, but cures to generate a polymer in the absence of air and in the presence of metal ions, as for example in a metallic bond-line.
Typical applications are the securing ofscrews and threads, the fixing ofadherents, ball bearings and bushes, and the sealing of surfaces. Anaerobic adhesives with improved resistance to temperature and the ability to take up oil are suitable for use in motors. Anaerobic adhesives are one-part systems that are easily and economically applied. The adhesive only cures in the bond-line, and noncured adhesive is easily removed. Owing to the low activation energy of the curing process, the polymerization takes place at room temperature. Since, in general, starting systems with higher reactivity are used, the polymers have a high degree of crosslinking and a very good resistance to different media and temperatures.
One prerequisite for a successful curing process is the presence of metal ions on the surface of the substrate, because they act as catalysts. Their influence on the curing rate depends on their degree of activity (copper > brass/bronze > iron > steel > zinc > aluminum > special steel). Typical starting monomers include triethylene glycol dimethacrylate or tetraethylene glycol dimethacrylate (Figure 5.26).
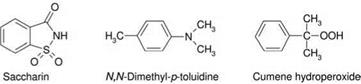
Tertiary amines, sulfimides or hydroperoxides are used as hardeners. A typical hardener system consists of saccharin, N, N-dimethyl-p-toluidine and cumene hydroperoxide (Figure 5.27).
|
Figure 5.26 Triethylene glycol dimethacrylate as a starting monomer. |
|
Figure 5.27 The components of the curing system. |
The radical curing of these adhesives is initiated by cumene hydroperoxide. Especially active metal ions are regenerated in a cycle by saccharin and amine, providing for a rapid and full cure (Figure 5.28).
Anaerobic adhesives are mainly used to take advantage of their chemical interlocking effect rather than their bonding effect, owing to the characteristic properties of their polymers. However, anaerobic adhesives may be impact-toughened to become suitable for surface-to-surface bonding in the case of very narrow gaps. When combined with UV-setting adhesives (see below), anaerobic adhesives can also be used for other applications.
 23 сентября, 2015
23 сентября, 2015  Pokraskin
Pokraskin 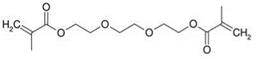
 Опубликовано в рубрике
Опубликовано в рубрике 