The reaction between epoxides and polar substances may take place in two different ways, both of which are associated with an opening of the oxirane ring.
Polymerization by Polyaddition In the manufacture of adhesives, the most important reaction type is the addition of a highly polar compound (hardener) to an epoxide with simultaneous ring opening (Figure 5.12) [25, 26].
|
Figure 5.12 Addition reaction of a secondary amine and an epoxy group. |
Often, primary or secondary amines are used as hardeners, thus generating a product that contains two groups with high polarities — that is, an amino group and a hydroxyl group which both promote the adhesive properties ofthe resin. Long-chain polymers are generated starting with diepoxides and diamines; this reaction is known as ‘polymerization by addition’ or, preferably, ‘polyaddition’.
Another group of widely used hardeners is that of liquid, low-molecular-weight polyamides which have amino groups at both ends of their molecules (polyaminoa — mides). These are relatively nontoxic and have only a slight inherent odor. Their reaction rate is a little slower than that ofamines, and the heat development less intense.
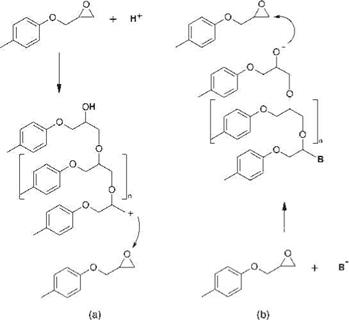
Ring-Opening Polymerization The second type of epoxide reaction is the so-called ‘ring-opening polymerization’ of the oxirane ring, initiated by a substance that activates opening of the oxirane ring by an addition reaction. The ring-opening reaction may be either acid-catalyzed or catalyzed by non-nucleophilic bases (Figure 5.13).
The initial ring opening then starts a chain reaction. In contrast to polyaddition, which requires a defined ratio of the reaction partners, ring-opening polymerization requires only a small amount of the initiator. The polymers thus generated have considerably fewer polar groups and hence less-favorable adhesion properties than those generated from diamines and diepoxides by polyaddition. Another disadvantage of the ring-opening polymerization reaction is that, after addition of the initiator, the reaction takes place very quickly and only a short time is left for processing. Therefore, in terms of the curing of epoxy adhesives polymerization by polyaddition is of minor importance. However, the process is used in cationic photocuring, where epoxy resins are mixed with materials that liberate cations when exposed to high — energy radiation (e. g. UV light). The ring-opening polymerization reaction is initiated by the liberation of cations.
Curing with Dicyandiamide Dicyandiamide is a colorless powder that is poorly soluble in liquid epoxy resins and, therefore, does not react with epoxy groups at
|
Figure 5.13 (a) Acid-catalyzed and (b) base-catalyzed ring-opening polymerization of epoxides. |
room temperature. Only at temperatures above 150 °C will dicyandiamide react, resulting in the curing of the epoxy resin. Hence, dicyandiamide — which is referred to as a ‘latent hardener’ — is admixed to the resin during adhesive manufacture to generate a one-part adhesive.
The reaction between dicyandiamide and epoxides begins with an addition reaction, the primary products of which initiate a ring-opening polymerization together with further epoxides. Dicyandiamide is a dimer of cyanamide, and in solution there exists an equilibrium between both compounds. Dicyandiamide acts as source of cyanamide in the reaction with epoxides; the cyanamide joins two molecules of epoxide to generate an aminooxazoline as a first intermediate. The amino group of this intermediate then adds to another epoxide to form a tertiary amine that acts as a catalyst for a subsequent base-catalyzed ring-opening polymerization (Figure 5.14) [27, 28].
The curing temperature of epoxides and dicyandiamides may be reduced to 125-130 °C by adding urea derivatives such as monuron or diuron.
5.5.3
 9 сентября, 2015
9 сентября, 2015  Pokraskin
Pokraskin 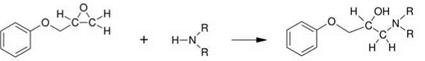
 Опубликовано в рубрике
Опубликовано в рубрике 