Although, for a long time, natural rubber [poly (1,4-cis-isoprene); see Figure 5.1] was used as base material for PSAs, today they are made from a great variety of elastomers.
In order to generate tack and adhesion, ‘tackifiers’ and other materials are mixed with the base material. These formulations stand out for their excellent adhesion features and, particularly when used as masking tapes in painting processes, their excellent removability after the paint curing process. PSAs based on natural rubber, however, have a tendency to yellow and crosslink, thus becoming brittle, due to the double bonds present in the base polymer. Although this problem was partly relieved by the addition of antioxidants, natural rubber-based PSAs remain unstable to longterm exposure to the environment. Several other base resins were therefore developed which did not suffer from these deficiencies [2].
During World War II, there was a shortage of natural rubber and therefore a number of new base resins had to be introduced. It had been known since 1929 [3] that alkyl acrylate ester polymers had the characteristic tack of PSAs, and a short time later it was discovered that polyvinyl alkyl ethers presented the same tacky feature. Due to the shortage of natural rubber, the development of these new materials experienced a rapid boom. Unlike the former formulations, they were transparent and colorless, and they had an excellent resistance to aging processes. However, the first formulations based on polybutyl acrylate and polyiso-butyl ether did not have the required tack and cohesive strength, respectively. However, after many years of successful development acrylic copolymers, for example, were widely accepted for use in high-performance PSA tapes (Figure 5.2).
The two primary acrylates used are 2-ethylhexyl acrylate and iso-octyl acrylate, but polymers based exclusively on these monomers do not perform as PSAs in their own right. During the early 1950s, various research groups found that the performance of these materials could be substantially improved by adding certain amounts of acrylic acid [4].
Generally, acrylate adhesives do not require any tackifiers to perform as well as PSAs; rather, their pressure-sensitive characteristics result from the inherent physical properties of the acrylate polymer. Today, acrylate-based PSAs are used in a wide variety of applications, from transparent tape to medical tape, office tape or foamed adhesive tape for industrial high-performance applications.
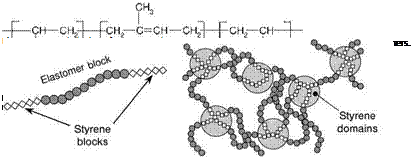
Both, natural rubber and acrylate-based PSAs must be chemically crosslinked in order to obtain the cohesive strength necessary to meet all of the requirements of a PSA. Block copolymer-based elastomers meet these requirements through phase separation. A-B-A block copolymers of isoprene with styrene (S-I-S) or butadiene with styrene (S-B-S) were introduced during the 1960s. The structure of a S-I-S block copolymer is illustrated in Figure 5.3.
Following coating, the glassy styrene blocks phase out and yield a phase-separated structure in the elastomer matrix due to incompatibility with the elastomer (i. e. polyisoprene or polybutadiene) (Figure 5.4), and this leads to physical crosslinking. Block-copolymer-based PSAs are used in a wide array of applications, but
—— ОН,—CR—
![]() г I COjR’
г I COjR’
 |
most commonly in packaging tapes. However, due to the double bonds in the B-block, these adhesives suffer from oxidative instability in the same way as natural rubber — based adhesives. In comparison to acrylate systems that achieve only about 50-80% of their final strength after pressure application but then build up final strength during the course of hours or days, block copolymer systems reach their final strength shortly after compression.
Since elastomers have a low tack and a low adhesion force towards commonly used surfaces, tackifiers must be added to natural rubber-based and A-B-A block copolymer-based adhesives. For this purpose, the viscous component is introduced into the elastic polymer. However, even if the base polymer has adequate viscoelastic properties to perform as well as a PSA, acrylates are tackified in order to improve adhesion on low-energy surfaces such as polyethylene or polypropylene. With regard to the base elastomer, a tackifier must fulfill the following requirements [5]:
• adequate compatibility
• very low molecular weight
• higher glass transition temperature (Tg)
Tackifiers increase the Tg-values of the PSAs and decrease their storage plateau modulus. As illustrated in Figure 5.5, tackifiers decrease the storage plateau modulus of elastomers to such an extent that the polymers fulfill the Dahlquist criterion for PSAs (see Section 5.1.5).
At present, the majority of tackifiers used have a low molecular weight and a Tg ranging from -20 °C to + 70 °C. At room temperature, they generally behave like brittle glass, and are classified into hydrocarbon — and natural resins-based products.
|
Figure 5.5 Influence of a tackifier on the storage modulus of a polymer. The Dahlquist criterion is discussed in Section 5.1.5. |
5.1.3
 30 августа, 2015
30 августа, 2015  Pokraskin
Pokraskin 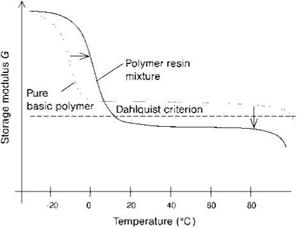
 Опубликовано в рубрике
Опубликовано в рубрике 