The classical pure silicones are made from hydrolysis of RSiX3, R2SiX2 and R3SiX, where R, in most cases, is methyl, phenyl or a mixture of both. If only methyl substituted “D” units are used, with few “M” units as chain stoppers, extremely nonpolar polydimethylsiloxanes, commonly known as silicone oils (Figure 2.70), are obtained. Their viscosity is governed by the D:M ratio. They are chemically inert, non-film forming products. Nevertheless, due to their low surface energy, they are extensively used as wetting additives, flow modifiers and defoamers.
|
Figure 2.70: Schematic representative structure of silicone oil and silicone resin |
Branched polysiloxanes are more useful as binders for coatings. The branching is introduced by the use of “T” units in the composition. The organic substitution may be methyl, phenyl or a mixture of both (Figure 2.70). Properties of the final resins are largely governed by the R:Si ratio (composition of M, D and T units), and methyl and phenyl content.
 25 октября, 2015
25 октября, 2015  Pokraskin
Pokraskin 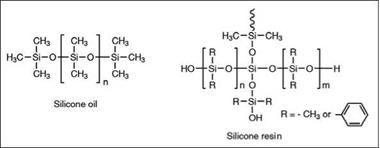
 Опубликовано в рубрике
Опубликовано в рубрике 