PUDs are another commercially important type of waterborne polyurethane systems. Due to their versatility, stability, high performance, and low VOCs, PUDs are increasingly used as binders for a wide range of coatings and related products. Conventional PUDs are aqueous secondary dispersions of high MW polyurethanes with a typical particle diameter of 0.1 to 0.2 pm. Such polyurethane resins are offered as translucent dispersions with ~35 to 45 % by weight solids. After application, PUDs form films by evaporation of water (and other volatiles) followed by particle coalescence, similarly to latex.
Typical thermoplastic PUDs are prepared by reaction of polymeric diols with diisocyanate, monomeric diols or diamines, called chain extenders, and a diol-containing hydrophilic group. The high MW polymer chains of such polyurethane resins contain soft domains arising from polymeric polyols and hard domains from isocyanates, chain extenders and hydrophilic groups containing a diol. A commonly used prepolymer process for preparation of PUDs is shown in Figure 2.68. The first step in PUD synthesis by this process is preparation of a — NCO functional prepolymer, generally in the presence of a polar aprotic solvent such as N-methyl-2-pyrrolidone, followed by neutralization of the carboxylic acid group using a volatile amine. The product is then dispersed in water under high shear conditions and the prepolymer MW is further increased by chain extension using diamine compounds. The higher reactivity of — NCO groups towards amines compared to water is taken advantage of in preventing excessive formation of urea linkages over urethane linkages. Nevertheless, such products always contain a distribution of urethane and urea linkages and hence, more specifically, such dispersions are called polyurethane-urea dispersions. The current trend is to offer solvent-free (typically N-methyl-2-pyrrolidone-free) dispersions because of VOC concerns. PUDs find a number of applications in coatings for wood, plastics, leather, and many more products.
Cross-linkable PUDs contain reactive functional groups pendant to the backbone and/or at the chain ends, and can be cured using a variety of cross-linkers and curing conditions. The reactive functionalities may include, among others, a fatty acid moiety in air-drying PUDs, — OH functionality for cross-linking with melamine or isocyanate type cross-linkers, — COOH groups for curing with carbodiimide
|
Figure 2.68: A simplified reaction scheme for preparation of an aqueous PUD using a prepolymer process |
|
Table 2.8: Types of polyurethane coatings
SB = solvent-borne, WB = waterborne, HS = high solid, PC = powder coating, SF = solvent-free |
or aziridine type cross-linkers, and (meth)acrylate functionality for UV-curing applications. In general, thermomechanical properties of cross-linked films of PUDs are much superior to those of thermoplastic films and hence find high-end industrial applications.
Table 2.8 summarizes different types of polyurethane coating systems, their compositions, curing mechanism and ASTM classification.
 23 октября, 2015
23 октября, 2015  Pokraskin
Pokraskin 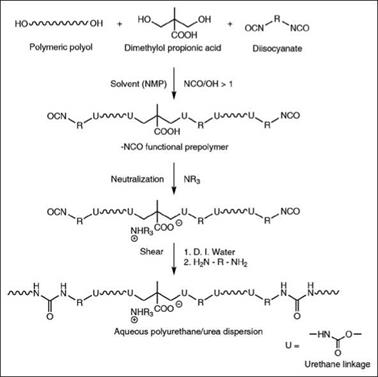
 Опубликовано в рубрике
Опубликовано в рубрике 