Acrylic resins are generally prepared by radical initiated chain — growth polymerization. As mentioned earlier in this chapter, chain — growth polymerization allows for preparation of polymers with very high MWs, of the order of a few hundreds of thousands of grams/ mole. Acrylic resins with such high MWs are generally capable of
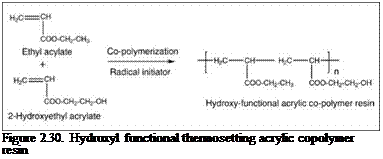
forming useful coatings under ambient conditions without the need for cross-linking. These resins, known as thermoplastic acrylics, are generally used in a wide range of architectural and specialty coatings. Resins prepared using one or more functional (meth)acrylate monomers will result in functionalized acrylic resins (Figure 2.30) that can be cross-linked through their pendant functional groups using appropriate cross-linkers. Such acrylic resins and coating systems are known as thermosetting acrylics. Thermosetting acrylics are widely used in high-end industrial coating applications such as automotive coatings and appliance coatings.
The two most important features of any acrylic monomer are: (a) its parent structure and (b) the type of alcoholic chain forming the ester group. As mentioned before, there are two primary types of acrylate monomer families: acrylate monomers and methacrylate monomers (see Figure 2.31). By virtue of their chemical structures and reactivity, the film properties of resins obtained from these
Figure 2.31: General structures of acrylates (acrylic acid esters) and methacrylates (methacrylic acid esters)
monomers are significantly different. In general, coatings based on methacrylate resins are much harder (have a higher Tg), less flexible and have better outdoor durability properties than their acrylic counterparts. Therefore, resins based on methacrylate monomers are used in applications requiring outstanding outdoor durability and chemical resistance.
The type of alcoholic part has a significant effect on thermo-mechanical properties, such as Tg, hardness, flexibility, impact resistance and adhesion of acrylic coatings. In general, as the number of carbon atoms in the alcoholic chain increases up to about 8, their film hardness, T viscosity and solvent resistance decreases while their compatibility with a wide range of resins and solvents increases. This is because the dangling chain of the pendant alcohol on the polymer backbone increases the free volume of the cured film. The increase in free volume brings about all the changes in their physicochemical and thermo-mechanical properties. Branched chain alcohols have more significant impact compared to their linear counterparts due to steric effects. Figure 2.32 illustrates the effect of an increase in number of carbon atoms of the alcoholic chain of the acrylic monomers on the Tg of their polymers.
Table 2.6 shows Tg values of some selected acrylic and vinyl homopolymers expressed in °C. Note the effect of type of alcoholic chain as well as parent monomer structure on the Tg of homopolymers. The
|
Figure 2.32: Effect of increasing chain length of alcoholic part on polymer properties |
Tg of acrylic copolymer resins depends on the Tg values of individual constituent monomers and their proportion in the chain. A particularly useful means of estimating the Tg of a copolymer is that given by the Fox equation:
vr (copolymer) = (x/Tgl)+(X2/Tg2)+(x/Tg3)+ .. . +(x/Tg[)
Where, xj is the weight fraction of the first monomer in the copolymer and Tgj is the homopolymer glass transition temperature of the first monomer. In this equation, Tg is the glass transition temperature expressed on the Kelvin scale.
|
Homopolymer |
Tg [°C] |
Homopolymer |
Tg [°C] |
|
Poly(acrylic acid) |
105 |
Poly(ethyl methacrylate) |
65 |
|
Poly(methyl acrylate) |
9 |
Poly(butyl methacrylate) |
22 |
|
Poly(ethyl acrylate) |
-24 |
Poly(2-hydroxyethyl acrylate) |
-15 |
|
Poly(butyl acrylate) |
-54 |
Poly(vinyl acetate) |
32 |
|
Poly(2-ethylhexyl acrylate) |
-70 |
Polyacrylonitrile |
110 |
|
Poly(methyl methacrylate) |
105 |
Polystyrene |
100 |
|
Table 2.6: Tg of some selected acrylic and vinyl homopolymers |
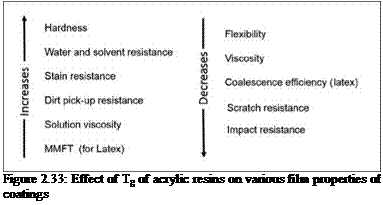
The effect of Tg of acrylic resins on various film properties is depicted in Figure 2.33.
Thus, it follows that proper selection of the types and amounts of monomers is critical in deriving acrylic copolymer resins with the correct Tg, which in turn controls many physic-chemical properties of their coatings. Besides Tg considerations, monomer selection also takes into account such important properties of coatings as outdoor durability, film clarity, gloss, gloss retention, film formation capability and emulsion stability.
 22 сентября, 2015
22 сентября, 2015  Pokraskin
Pokraskin 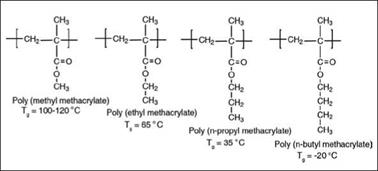
 Опубликовано в рубрике
Опубликовано в рубрике 