Phenolic resins are prepared by condensation of phenolic compounds with aldehyde, commonly formaldehyde. Phenol was initially predominantly used to prepare phenolic resins, but subsequently, a greater variety of alkyl or aryl substituted phenols were explored to tailor the properties of resins by controlling functionality to meet diverse requirements of different coatings. An alkyl group substi-
|
|
tuted at the para position increases color stability, and increasing the number of carbon atoms in the alkyl group improves oil and hydrocarbon solubility. Along with various petrochemical derived phenols, some phenolic compounds of plant origin, such as cashew nut shell liquid and its derivative cardanol, are also used widely. A few representative phenols are shown in Figure 2.18.
Formaldehyde is the most common aldehyde used for making phenolic resins. Formaldehyde, being a gas, is normally used as a 37 to 41 % aqueous solution (formalin) or as a solid in polymeric form (paraformaldehyde) that thermally decomposes to formaldehyde during reaction. For some special purposes, furfural or another complex aldehyde is also used.
 17 сентября, 2015
17 сентября, 2015  Pokraskin
Pokraskin 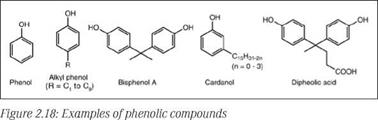
 Опубликовано в рубрике
Опубликовано в рубрике 