Unsaturated polyester resins are low MW condensation polymers which are transformed to a cross-linked network via radical initiated polymerization. The double bonds in the backbone copolymerize with unsaturated monomer (reactive diluent) present in the system. During polymerization, relatively short low MW polyester chains are cross-linked by short bridges consisting of, on average, around two to three styrene units, forming a densely cross-linked polymer network (see Figure 2.17).
|
|
Free-radical initiated copolymerization can be accomplished either by conventional initiation with organic peroxides or hydroperoxides or phytochemically by using a photoinitiator in UV light. A detailed discussion of UV cured systems is outside the scope of this section; therefore, only conventional initiation is discussed here. The conventional curing of unsaturated polyester resins can proceed either by thermal curing or under ambient conditions using redox system accelerators and promoters, in addition to initiators. The latter approach is more common in the coating industry as two-pack systems where initiator solution or paste is supplied separately, while accelerators and promoters are normally mixed with the unsaturated polyester.
Examples of some important initiators are ethyl methyl ketone peroxide, cyclohexanone peroxide, benzoyl peroxide and cumene peroxide. Accelerator is a reducing agent, such as cobalt octoate, which is added in very small quantity to catalyze decomposition of the initiator into free radicals. Aromatic amines such as dimethyl aniline or dimethyl p-toluidine are added to promote that reaction. The two components are combined prior to application, ensuring even distribution of initiator in the system. The dosage of initiator, accelerator, promoter and inhibitor will determine the pot life, the longest period of time during which mixture is still usable and can be applied.
Air inhibition of the curing reaction is one of the major limitations of unsaturated polyester systems. Polymerization is considerably inhibited by oxygen, which is added to the terminal radical of growing polystyrene chains by forming a stable peroxide radical. In most coating applications, the top surface is exposed to the air and will remain tacky while the layers below are cured. This is normally addressed by incorporating some insoluble semi-crystalline paraffin wax in the formulation to minimize the problem. Once the coating is applied, the low surface tension wax particles preferentially migrate to the surface and provide a barrier between the oxygen and polymerizing coating, thereby minimizing the difficulty of surface cure. It also reduces the rate of styrene loss from the coating. However, it has tendency to reduce the gloss of the surface, and therefore, for high gloss finishes, the surface needs to be polished after application.
In another approach, to minimize air inhibition, reactive oxygen species are added to the system, which preferentially consume oxygen before it can interfere with the curing reaction. Allyl ethers such as trimethylolpropane diallyl ether, pentaerythritol monoallyl ether and allyl glycidyl ether are normally used for this purpose.
 14 сентября, 2015
14 сентября, 2015  Pokraskin
Pokraskin 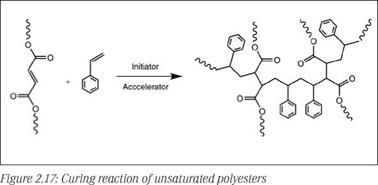
 Опубликовано в рубрике
Опубликовано в рубрике 