Direct esterification is one of the most important routes for forming saturated polyesters, and involves condensation of polybasic acids and polyols with removal of water of the reaction. Another important reaction of this type is self-condensation of polyfunctional monomers having both hydroxyl and carboxylic acid group.
Transesterification between polyols (generally diols) and dialkyl esters of dicarboxylic acids (generally methyl esters) can also lead to polyesters. This is an important method for deriving powder coating resins with terephthalic acid and isophthalic acid. The generic reactions involved in direct esterification and transesterification were discussed in Section 2.3.1.
Ring opening polymerization of lactones (cyclic esters) is another interesting technique to derive polyesters. For instance, lactones are reacted with polyols (as an initiator) to derive a linear polyester (polylactone) by ring opening polymerization as shown in Figure 2.13. One interesting aspect of this technique is that the functionality of the final polymer is the same as that of the initiator polyol.
|
Figure 2.13: Representative scheme for ring opening polymerization |
 7 сентября, 2015
7 сентября, 2015  Pokraskin
Pokraskin 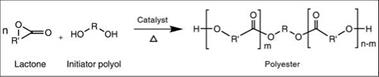
 Опубликовано в рубрике
Опубликовано в рубрике 