Natural oils, used as components of binders for surface coatings, are generally derived from oilseeds, though occasionally they are of animal origin, such as fish oil. Compositionally they are triglycerides of fatty acids. They are sometimes described as fixed oils to distinguish them from essential oils, which are volatile aromatic oils found in some plants. The physical and chemical properties of triglyceride oils are governed by the composition of fatty acids in them. The generic structure of a triglyceride oil is shown in Figure 2.5.
|
|
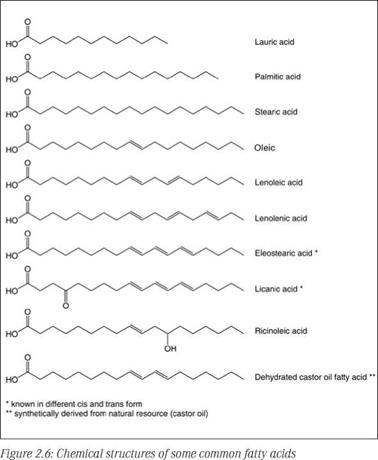
R, R’ and R’’ are fatty acid chains, which may or may not be of one type in a given triglyceride molecule. The fatty acid distribution is broad and characteristic in specific plant oils. Some of the important natural fatty acids commonly found in vegetable oils are shown in Figure 2.6.
Three important attributes of the structure of these fatty acids are: The length of the fatty chain, the degree of unsaturation (number of double bonds) of the chain and the position of the double bonds in polyunsaturated chains.
The chain length of fatty acids can vary from C8 to C22, but C18 is the most common fatty acid in the case of vegetable oils. Fish oil is the only animal oil that has an even broader range of fatty chain length, from C12 to C30 with variation in unsaturation. The saponification value of triglyceride oils is an indirect measurement of their chain length.
The double bonds in the chain are the reactive centers responsible for thermal and oxidative polymerization properties of the oils. A quantitative measure of the average degree of unsaturation present in a given oil is expressed by its iodine value (IV), which increases with an increasing number of double bonds. Therefore, highly unsaturated oils having the ability to form a solid or semisolid polymeric structure by means of oxidative or thermal polymerization are invaluable for coatings.
In addition to the number of double bonds, their placement in the fatty chain with respect to each other also has a considerable influ-
|
|
ence on properties of oils. Based on this, oils are classified as conjugated or unconjugated; in the former, two or more double bonds are arranged to give a system of alternating single and double bonds (-CH=CH-CH=CH-), while in the latter, double bonds are separated by two or more single bonds (-CH=CH-CH-CH=CH-). A conjugated molecule is much more reactive than an unconjugated one with the same degree of unsaturation. This is the reason for the reactivity of dehydrated castor oil (a conjugated diene) and the extreme reactivity of tung oil (conjugated triene), compared to, for instance, soybean oil (an unconjugated oil).
Certain oils contain characteristic fatty acids, such as ricinoleic acid in castor oil, with hydroxyoleic acid (12-hydroxy-9-octadecenoic acid); eleostearic acid in tung oil, with a conjugated triene; licanic acid in oiticica oil, with a conjugated triene keto acid, which are responsible for the high reactivity of the parent oils.
Raw oils are obtained from oilseeds by solvent extraction or by mechanical pressing. They contain a variable amount of non-glyceride impurities that are not desirable for their applications in paints and coatings. Therefore, refined oils with considerably low odor, color and impurities are commercially supplied for use in coatings and related industries.
 26 августа, 2015
26 августа, 2015  Pokraskin
Pokraskin 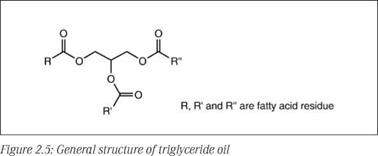
 Опубликовано в рубрике
Опубликовано в рубрике 