Rosin, also known as colophony, and its derivatives are among the most important and widely used natural resins. Rosin is obtained from pine trees either by collecting exudations from the tree (known as gum rosin) or by solvent extraction of aged pine stumps (known as wood rosin). It can also be obtained as a by-product of the paper industry, where soluble salts of rosin and fatty acid along with lignin are formed as a by-product, which is further distilled to obtain fatty acid and rosin as a residue (known as tall oil rosin). Chemically, rosin is mixture of monocarboxylic acids (~90 %), with the main component being abietic acid and the remainder (~10 %) being neutral materials such as hydrocarbons, oxidized terpenes and saponifiable esters (see Figure 2.4). When heated at ~150 °C, abietic acid isomerizes to levopimaric acid. The unsaturated abietic acid can be readily oxidized, but levopimaric acid is more resistant to oxidation. Rosin is a light yellow to brown brittle solid material (softening point ~70 to 80 °C) having good solubility in aliphatic solvents and good compatibility with drying oils. But due to its high acid content, it is sensitive to water and alkali. Therefore, rosin is frequently chemically modified to increase its softening point and lower its acidity. The important chemical modifications of rosin involve either neutralization of acid groups with calcium oxide (lime) or zinc oxide to form calcium or zinc soaps, respectively, or its esterification with polyols such as glycerol or pentaerythritol for necessary molecular enlargement to the product commonly known as ester gum. The carboxylic acid group of rosin acid is sterically hindered and hence requires a higher temperature (~250 °C) for esterification, but on the other hand, its esters have better resistance to hydrolysis.
Maleic modified rosin is a commercial product and widely used as a binder in the printing ink industry. Levopimaric acid with its conjugated double bonds can undergo a Diels-Alder reaction with maleic anhydride, maleic acid or fumaric acid to produce maleic modified rosin adduct, which is a tricarboxylic acid derivative. The adduct is then reacted with a polyol such as glycerol or pen — taerythritol to produce maleic resins, which are characterized by their light color, higher melting point, improved light fastness, better hardness and oxidation resistance than rosin. A wide range of such products are commercially available, mainly varying in softening point and excess hydroxyl content.
Rosin modified phenolic resin, another important rosin derivative used in the coating industry, is derived by reacting rosin with a resole type phenolic resin. The extent of modification, the type of phenolic resin used (in terms of the phenolic compound used), and
|
Figure 2.4: Schematic representation of some modifications of rosin |
the formaldehyde to phenol (f:p) ratio are the main variations offering different grades of these products. Please see Section 2.6.3 for further discussion of this topic.
The main applications of rosin and its derivatives are in printing inks, oleoresinous varnishes, and modified alkyds to improve hardness and physical drying.
 23 августа, 2015
23 августа, 2015  Pokraskin
Pokraskin 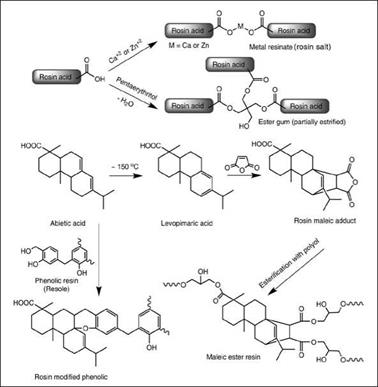
 Опубликовано в рубрике
Опубликовано в рубрике 