 |
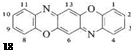
Dyes derived from the triphenodioxazine ring system (18) have been commercially available since 1928 when Kranzlein and coworkers discovered dyes with this basic structure augmented by sulfonic acid groups. The unsubstituted triphenodioxazine (which is of no importance as a colorant) was first obtained by G. Fischer in 1879 [39], and its structure 18 was elucidated in 1890 [40].
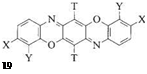 |
By varying the substituents on the orange parent substance 19, particularly in the positions para to the imino groups, its color can be modified. Red to red-violet shades result when X=OH or O R, and blue colors when X=NH2 or NHR’. A blue shade also results if the external phenyl groups of the dioxazine system are part of an annulated and highly condensed aromatic ring system, for example, pyrene (C. I. DirectBlue 109, [33700-25-3]).
|
Table 3.1 Absorption maxima of triphenodioxazines (19) as a function of substituent groups
|
Table 3.1 summarizes observed absorption maxima as a function of various substituents. In particular, blue triphenodioxazines have very high molar extinctions є, comparable to those of bisazo and phthalocyanine dyes. Until recently, anthraquinone dyes (є ca. 15 000) were predominant in most applications requiring brilliant blue dyes, but the much stronger triphenodioxazine dyes now represent a less expensive alternative in many applications.
Dioxazine chromophores show a sensitivity to acid and base that is more pronounced in dye solutions than in the fixed dyes. Electron-withdrawing substituents enhance this effect, a major reason why red reactive dyes based on tripheno- dioxazine have not yet been commercialized [41] even though they are described in most dioxazine patents. Much research and development effort has succeeded in suppressing this sensitivity in the blue dye products currently on the market. Generally, triphenodioxazine chromophores show a high degree of agglomeration and thus increased substantivity because of their planar arrangement. A higher sub — stantivity causes problems during dyeing, such as unsatisfactory leveling in the exhaust process, tailing from dark to light in the padding process, or limited removal of unfixed dye in the rinsing process. Studies have been directed toward overcoming these problems either with new products displaying less critical substantivity or by control of the substantivity through more suitable dyeing conditions.
The first commercial products of this type were sold as blue direct dyes with unmatched tinctorial strength, brilliance, and high lightfastness. An example is C. I. DirectBlue 106, 51300 [6527-70-4] (20):
|
|
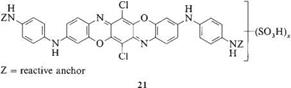
The triphenodioxazine chromophore was tested in almost every class of dyes, but only recently has it been introduced into reactive dyes. Dioxazines were investigated in some of the earliest work on reactive dyes in the late 1950s, and they are included in some of the first patents covering reactive anchors and reactive dyes [42]. In particular, compound 21 was invoked as a chromophore, with reactive anchors attached to the free amino groups of the molecule.
|
|
The first mixed azo — triphenodioxazine dye containing a monochloro-s-triazine group emerged in 1953 [43], with the triazine residue serving as a link between the two chromophores. In the years that followed, the dioxazine chromophore was also mentioned in most of the patents related to new reactive systems, although none led to a commercial product. At the beginning of the 1970s, the major activity seemed to be concentrated, leading to several exclusive patents for triphenodioxazine dyes [44]. Within a few years, the first commercial triphenodioxazine reactive dye was introduced: C. I. Reactive Blue 163, [72847-56-4]. Other products followed, all designed to achieve brilliant blue shades that could compete with the then-dominant anthraquinone chromophores in certain market segments [45].
The launching of these new products initiated research by other dye manufacturers. This led to numerous new dioxazine reactive dyes (Examples see Table 2.3).
 13 сентября, 2015
13 сентября, 2015  Pokraskin
Pokraskin 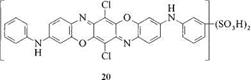
 Опубликовано в рубрике
Опубликовано в рубрике 