Sulfur dyes are synthesized by heating aromatic or heterocyclic compounds as amines, phenols, or nitro compounds with sulfur or, more usually, alkali metal polysulfides. Unlike most other dye types, it is not easy to define a chromogen for the sulfur dyes. It is likely that the chromophore of Sulfur Bake and Polysulfide Bake Dyes consists of macromolecular structures of the thiazole type 1, if the starting material contains amino and methyl groups. In those structures the sulfur is present as (sulfide) bridging links and thiazole groups (Scheme 2.6). Nothing is known about the structure of Sulfur Bake and Polysulfide Bake Dyes which are unable to form thiazole rings. These include, for instance, dyes, which are formed in the sulfur melt from decacyclene.
The characteristic properties of Polysulfide Melt (Chinonimin-) Dyes can be expressed to a large extent in a simplified model (see Scheme 2.8, p. 83).
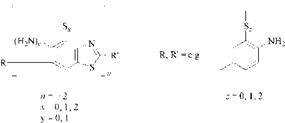 |
Scheme 2.6
Even now, knowledge of the constitution of sulfur dyes is rather fragmentary. Two reasons are that the preparation process yields complicated mixtures of related compounds and that sulfur dyes could not be obtained in pure form because of their amorphous and colloidal structure and their insolubility in common solvents.
 1 сентября, 2015
1 сентября, 2015  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 