The term phthalocyanine was first used by R. P. Linstead in 1933 [1] to describe a class of organic dyes, whose colors range from reddish blue to yellowish green. The name phthalocyanine originates from the Greek terms naphtha for mineral oil and cyanine for dark blue. In 1930-1940, Linstead et al. elucidated the structure of phthalocyanine (H2Pc) and its metal complexes [1-11]. The basic structure is represented by phthalocyanine (1) itself:
|
|
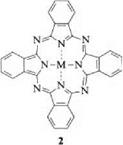
Phthalocyanine forms complexes with numerous metals of the Periodic Table. A large number of complexes with various elements are known [12-18]. Metal phthalocyanines MPc (2) and compounds with metalloids such as B, Si, Ge, and As or nonmetals such as P display a wide variety in their coordination chemistry.
|
|
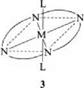
The coordination number of the square-planar complexes of Cu, Ni, or Pt is 4. Higher coordination numbers of 5 or 6 with one or two additional ligands such as water or ammonia result in square-based pyramidal, tetrahedral, or octahedral structures (3) [19-22].
|
|
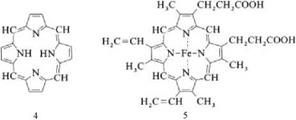
The phthalocyanines are structurally related to the macrocyclic ring system porphyrin (4). Formally, phthalocyanine can be regarded as tetrabenzotetraaza — porphyrin and as the condensation product of four isoindole units.
|
|
The phthalocyanines are structurally similar to naturally occurring porphyrins such as hemoglobin (5), chlorophyll a, and vitamin B12. Phthalocyanines themselves do not occur in nature.
History. Braun and Tschernak [23] obtained phthalocyanine for the first time in 1907 as a byproduct of the preparation of o-cyanobenzamide from phthalimide and acetic anhydride. However, this discovery was of no special interest at the time. In 1927, de Diesbach and von der Weid prepared CuPc in 23 % yield by treating o-dibromobenzene with copper cyanide in pyridine [24]. Instead of the colorless dinitriles, they obtained deep blue CuPc and observed the exceptional stability of their product to sulfuric acid, alkalis, and heat. The third observation of a phthalocyanine was made at Scottish Dyes, in 1929 [25]. During the preparation of phthalimide from phthalic anhydride and ammonia in an enamel vessel, a greenish blue impurity appeared. Dunsworth and Drescher carried out a preliminary examination of the compound, which was analyzed as an iron complex. It was formed in a chipped region of the enamel with iron from the vessel. Further experiments yielded FePc, CuPc, and NiPc. It was soon realized that these products could be used as pigments or textile colorants. Linstead et al. at the University of London discovered the structure of phthalocyanines and developed improved synthetic methods for several metal phthalocyanines from 1929 to 1934 [1-11]. The important CuPc could not be protected by a patent, because it had been described earlier in the literature [23]. Based on Linstead’s work the structure of phthalocyanines was confirmed by several physicochemical measurements [26-32]. Methods such as X-ray diffraction or electron microscopy verified the planarity of this macrocyclic system. Properties such as polymorphism, absorption spectra, magnetic and catalytic characteristics, oxidation and reduction, photoconductivity and semiconductivity, solubility, and photochemical and dielectric properties were investigated from the 1930s to the 1950s.
Copper phthalocyanine was first manufactured by ICI in 1935, where its production from phthalic anhydride, urea, and metal salts was developed [33]. Use of catalysts such as ammonium molybdate improved the method substantially [34]. In 1936, I. G. Farbenindustrie began production of CuPc at Ludwigsha — fen, and in 1937 Du Pont followed in the United States. The most important of the phthalocyanines, CuPc, is now produced worldwide. The first phthalocyanine dye was a phthalocyanine polysulfonate [25]. Other derivatives, such as sulfonyl chlorides, ammonium salts of pyridyl phthalocyanine derivatives, sulfur and azo dyes, and chrome and triazine dyes, have been patented since 1930. At that time, the use of phthalocyanines as colorants for printing ink, paint, plastics, and textiles began. Of the industrial uses, the application of CuPc in printing inks is its most important use. The greenish blue CuPc shade is suitable for color printing. Other favorable properties such as light, heat, and solvent resistance led to the use of this blue pigment for paints and plastics. The chloro and bromo derivatives are important green organic pigments. Other derivatives are used in textile dyeing and printing or for the manufacture of high-quality inks (pastes for ballpoint pens, ink jets, etc.).
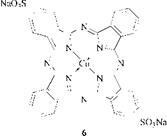
Of all the metal complexes evaluated, copper phthalocyanines give the best combination of color and properties and consequently the majority of phthalocyanine dyes (and pigments) are based on copper phthalocyanines; C. I. Direct Blue 86 (6) is a typical case.
As well as being extremely stable, copper phthalocyanines are bright and tinctorially strong (£max ca. 100 000); this renders them cost-effective.
|
|
 29 августа, 2015
29 августа, 2015  Pokraskin
Pokraskin 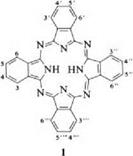
 Опубликовано в рубрике
Опубликовано в рубрике 