Glenz and Beckmann [24] were the first to investigate color balance and surface potentials in polyacrylonitrile fibers. On the basis of their results, they proposed the following dyeing mechanism: The cationic chromophore is first absorbed by the negatively charged fiber surface and then diffuses, at elevated temperature, into the interior of the fiber; there it binds to active acid groups, the number of which is limited and the accessibility of which depends on temperature and fiber constitution. Therefore, the dyeing characteristics of a cationic dye are determined by affinity and diffusibility.
The affinity of cationic dyes is enhanced by increasing the size of the molecule and especially by introducing aromatic residues. The phenyl-substituted dye 28 is absorbed onto polyacrylonitrile materials at a higher rate than the dye 11 [25].
|
|
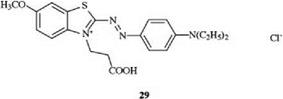
Residues which make the substance more hydrophilic, such as carboxyl and hydroxyethyl groups, lower the affinity of cationic dyes for polyacrylonitrile. Therefore, dye 29 is absorbed onto polyacrylonitrile materials much more slowly than dye 2 [26].
|
|
High affinity may, however, lead to problems in leveling, because these dyes are bound quickly and nearly irreversibly to the acid groups of the fiber.
Diffusibility increases with decreasing molecular mass. Dyes with a cation mass of less than 275 migrate well and are suitable for the production of level dyeings, especially in the lighter shades [27].
Cationic dyes are also used to dye polyacrylonitrile fibers during the spinning process either from dimethylformamide solution or in the gel stage subsequent to aqueous spinning processes.
In Western Europe, the bulk of acrylic fibers is targeted for the clothing industry. As much as two-thirds of acrylic fiber production is used in this sector, whereas only one-third is used for home furnishings and in technical applications.
 26 августа, 2015
26 августа, 2015  Pokraskin
Pokraskin 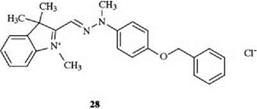
 Опубликовано в рубрике
Опубликовано в рубрике 