The most important coupling components can be divided into the following groups. Where there are several possibilities for coupling, the preferred coupling positions are marked by bold arrows and the other possible coupling positions by ordinary arrows.
 |
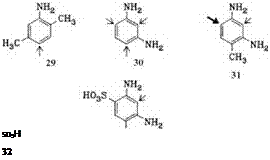 |
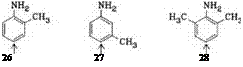
Anilines, diamin obenzenes (e. g., 26-32).
When treated with aniline, diazotized aniline (benzenediazonium chloride) yields, apart from a small quantity of 4-aminoazobenzene (33), diazoaminoben — zene (34) as the main product:
33 34
4-Aminoazobenzene (33) is obtained by coupling in a more strongly acid medium, but better still by heating 34 in aniline with addition of aniline hydrochloride.
Electron-donating substituents in the aniline, such as methyl or methoxy groups, especially at the meta position, promote coupling; the tendency to couple thus increases in the order aniline< o -toluidine< m — toluidine< m -anisidine<cresi — dine<1-amino-2,5-dimethoxybenzene (aminohydroquinone dimethyl ether) to the extent that without formation of the diazoamino compound, the last three bases are attached almost quantitatively in the desired position, i. e., at the 4-position relative to the amino group.
m — Phenylenediamines couple to form monoazo dyes (chrysoidines) or disazo dyes.
Naphthylamines, Naphthylaminesulfonic Acids. Some examples (35-38) are mentioned here; other compounds can be found among the diazo components (see Figure 2.2), because most naphthylaminesulfonic acids can be employed both for diazotization and for coupling purposes.
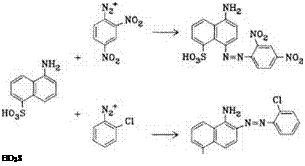 |
Whereas |3 — naphthylaminesulfonic acids always couple at the adjacent a position, in a-naphthylaminesulfonic acids, the coupling location is influenced by the position of the sulfonic acid group: 1,6-, 1,7-, and 1,8-naphthylaminesulfonic acids couple at the 4-position; 1,5-naphthylaminesulfonic acid (Laurent acid) only couples with very strong couplers (e. g., 2,4-dinitroaniline), mainly at the 4-position. With diazotized aniline, chloroaniline, or diazotized anilinesulfonic acids the 2- aminoazo dyes are obtained, but with diazotized nitroaniline, mixtures of the 2- and 4-coupling products form (Scheme 2.1).
Scheme 2.1
Phenols, Naphthols (e. g., 39-47). Phenols mainly couple at the 4-position, or at the 2-position if the 4-position is occupied. p-Hydroxybenzoic acid couples with elimination of CO2; resorcinol couples twice: initially at the 4-position, and with a second equivalent of diazonium compound at the 2-position under acid conditions or at the 6-position under alkaline conditions. a-Naphthols mainly couple at the 4-position, in addition to which varying quantities of 2- and 2,4-coupling products are obtained, depending on the diazo component. |3-Naphthol couples at the 1-position. Substituents in the 1-position, such as SO3H, COOH, Cl, CH2OH, or
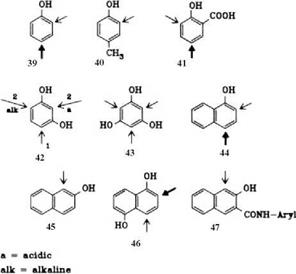 |
CH2NR2, may be eliminated during the coupling process. 1-Methyl-2-naphthol does not form an azo dye.
Coupling components in this series that are of particular industrial importance are 2-hydroxynaphthalene-3-carboxylic acid arylamides in particular anilides, which also couple at the 1-position.
Phenols and naphthols generally couple more easily and rapidly than amines; phenol-3-sulfonic acid can be coupled, but not aniline-3-sulfonic acid.
The readiness of the phenols to couple increases with increasing number of hydroxyl groups, for example, in the following series:
он он он OH
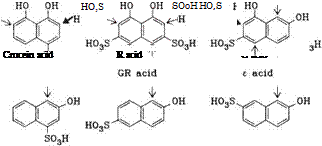 |
Naphtho Isulfonic Acids (Figure 2.3).
Chromotropic acid Dioxy G acid
Figure 2.3
1-Naphtholsulfonic acids mainly couple at the 2-position. The 4-coupling products obtained as byproducts must be carefully removed from the azo dyes, because unlike the 2-substitution products, their shade changes as a function of the pH value (shade intensification with rising pH due to formation of phenolate or naphtholate resonance structures).
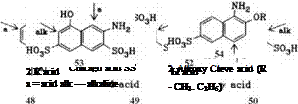 |
Amino phenols, Aminophenolsulfonic Acids, Aminonaphtholsulfonic Acids (e. g., 48-54).
In alkaline medium m-aminophenol couples at the position para to the hydroxyl group; in an acid solution p-hydroxy and p-aminoazo dyes are obtained.
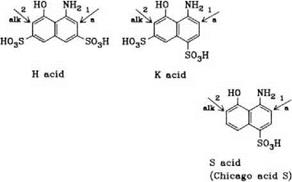
In aminonaphtholsulfonic acids such as H, K, and S acids, orientation is strongly influenced by the pH of the coupling medium. In an acid medium the azo group enters the position ortho to the amino group, and in an alkaline medium, in the position ortho to the hydroxyl group. 1-Amino-8-naphtholsulfonic acids with free ortho positions can couple with two equivalents of diazonium compound, coupling initially taking place in the acid medium at the position ortho to amino group, followed by coupling in the alkaline range at the position ortho to hydroxy group. Aminonaphtholsulfonic acids (o-hydroxyazo dyes) initially coupled in an alkaline medium cannot normally undergo further coupling to form disazo dyes. However, if this initial coupling is followed by a metal-com — plexation step, further coupling is possible in the neutral or alkaline range.
Compounds with Reactive Methylene Groups. In this group the coupling components with the greatest industrial importance are the N-acetoacetyl derivatives of aromatic amines (acetoacetarylides) CH3COCH2CONHAr. Anilines substituted by halogen, alkyl, alkoxy, nitro, and acylamino groups are most suitable (e. g., Napthol AS-IrG).
h„co
![]()
 |
CH, COCH„CONH
OCH.,
Naphtol AS-IRG
 |
Heterocyclic Components. The most important compounds in this range are the 5- pyrazolones substituted at the 3-position.
Further examples are (55)-( 61).
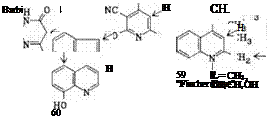 |
8~HydroxyqumoUne 2,4-Dihydroxyquinoline
Coupling components containing both amino and hydroxyl groups, such as H acid (1-amino-8-naphthol-3,6-disulfonic acid) can be coupled stepwise (Scheme 2.2). Coupling is first carried out under acid conditions to effect azo formation in the amino-substituted ring. The pH is then raised to ionise the hydroxyl group (usually to pH>7) to effect coupling in the naphtholate ring, with either the same or a different diazonium salt. Performing this process in the reverse order fails because the nucleophilicity of the amino group is insufficient to facilitate the second coupling step.
The unusual conditions needed to produce an azo dye, namely, strong acid plus nitrous acid for diazotization, the low temperatures necessary for the unstable diazonium salt to exist, and the presence of electron-rich amino or hydroxy compounds to effect coupling, means that azo dyes have no natural counterparts.
|
Scheme 2.2 |
 20 августа, 2015
20 августа, 2015  Pokraskin
Pokraskin 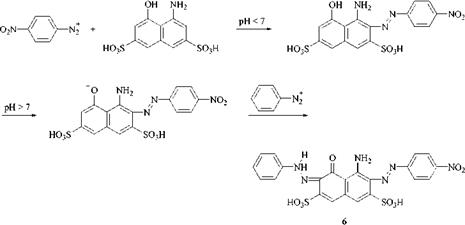
 Опубликовано в рубрике
Опубликовано в рубрике 