5.5.4.1
Optical Excitation of Luminescence and Energy Transfer
When absorption of UV or even visible light leads to emission, one speaks of optical excitation of luminescence. This process takes place in e. g. fluorescent lamps and phosphor-converted LEDs in which phosphors are used to at least partly change the wavelength of the radiation emitted by the LED. Optical absorption can take place on the already discussed impurities (optical centers), these being either the activator ions or sensitizer ions. Sensitizer ions are used when the optical absorption of the activator ions is too weak (e. g. because the optical transition is forbidden) to be useful in practical devices. In such a case, energy transfer from the sensitizer ions to the activator ions has to take place. The optical absorption leading to emission can also take place by the host lattice itself (band absorption). In this case one speaks of host lattice sensitization. Energy transfer from host lattice states to the activator ions (in some cases also involving sensitizers) has to take place.
In the blue emitting luminescent material BaMgAl10O17:Eu both the absorption and the emission process originate from optical transitions between the 4f and 5d levels of the Eu2+ ion. As the transition leading to optical absorption is allowed, a relatively small Eu2+ concentration is sufficient to adjust a sufficiently strong absorption in practical devices. The excitation spectrum of BaMgAl10O17:Eu is given in Figure 5.51.
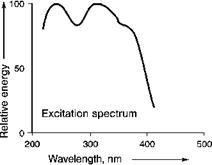 Fig. 5.51 Excitation spectrum of the Eu2+ emission in BaMgAl10O17.
Fig. 5.51 Excitation spectrum of the Eu2+ emission in BaMgAl10O17.
One observes, a strong broad absorption spectrum in the UV part of the spectrum as the excited 5d state of the Eu2+ ion is split by ligand field interaction with the oxygen ions surrounding it.
The excitation spectrum of the Mn2+ spectrum in BaMgAl10O17:Eu, Mn is, in the UV, very similar to the excitation spectrum of the compound without Mn2+. This is an example of Eu2+-sensitized emission of Mn2+, as proven by the similarity of the excitation spectrum of both the Eu2+ and the Mn2+ emission. The very localized excitation (exciton) of Eu2+ is transferred to the Mn2+ ion. The energy transfer process might involve more than one Eu2+ ion (excitonic energy transfer via the Eu2+ sublattice).
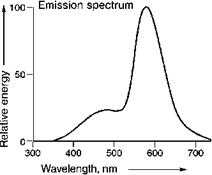 |
Mn2+ emission can also be sensitized by other ions, like Sb3+ in the well-known white emitting material Ca5(PO4)3(F, Cl):Sb, Mn. Here, orange emission is generated by Mn2+ and blue emission by the Sb3+. This material is applied widely in fluorescent lamps. Its emission spectrum is given in Figure 5.52.
It has to be noted that the emission spectrum depends on the Sb3+ and Mn2+ concentrations. By adjusting these concentrations, the color of the emission can be varied.
Another well-known sensitizer-activator pair is the Ce3+-Tb3+ couple (see Figure 5.47). All green emitting phosphors applied in high quality fluorescent lamps are based on this combination.
5.5.4.2
 21 января, 2016
21 января, 2016  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 