Reduction with Hydrosulfite. The reprecipitated dye (30 grams) is dissolved in 250 cc. water containing enough soda to make the solution distinctly alkaline to litmus. Hydrosulfite is added in small portions to the boiling solution until it becomes colorless. An excess of hydrosulfite is to be avoided or sulfur may separate.
The yellowish brown oil which separates from the yellow reduction solution solidifies on cooling. It is recrystallized twice from aqueous alcohol, using decolorizing carbon, yielding a base melting at 68°C. The filtered reduction solution is acidified and treated with salt, and after a short time a voluminous white precipitate is formed. This is filtered off and washed with salt solution. From 50 grams of the commercial product, 9 to 10 grams of purified base and 15 grams of the sulfonic acid are obtained.
The base has the following properties: It dissolves in dilute hydrochloric acid only on warming, and, on cooling the solution, a hydrochloride separates which melts at 168-170°C. and which is hydrolyzed by water. If the hydrochloric acid solution is heated to about 80°, partial decomposition occurs, producing an oil volatile with steam. The base contains halogen, but not sulfur. Nitrogen and halogen determination give a 1:1 ratio of chlorine to nitrogen and a molecular weight of 219. The acetyl derivative melts at 166°.
The salted-out sulfonic acid can be diazotized and coupled, but is very stable otherwise. Its properties suggest N-acylated H or К acid.
Reduction with Stannous Chloride. A solution of 20 grams of the purified dye in 250 to 300 cc. water is heated to boiling in a round-bottomed flask fitted with reflux condenser and stirrer. A solution of 40 grams of stannous chloride in 100 cc. concentrated hydrochloric acid (1.19) is added and the mixture is boiled for 3 hours. If the solution is still not completely decolorized, more of the stannous chloride solution is added.
The tin is now removed electrolytically from the reduction mixture. The liquid is placed in an acid-resistant clay cell of 350-cc. capacity and the latter is placed in a porcelain or Pyrex beaker filled with 10 per cent sulfuric acid to the same level as the liquid in the cell. The tin is plated out on a copper gauze electrode at a temperature of 80-90°, using a carbon rod as anode. At an E. M.F. of 8 volts and a current of 6 to 8 amperes, all of the tin used to reduce 20 grams of dye is removed in 4 to 5 hours. Electrolyzing is continued until hydrogen begins to be evolved. Nothing crystallizes from the detinned solution, so it is evaporated in vacuum to half its volume. On cooling, a light gray powder comes out. This material exhibits the reactions of 7-amino-H acid. The spectrum of the oxidized material has the following bands: Л,=530and490 m/* (from 7-amino-H acid, Л = 528 and 491 m^)
The filtrate, which smells strongly of toluenesulfonyl chloride, is evaporated to dryness. The gray residue is treated with soda solution, and the solution is shaken out with ether. From the ether extract, a substance crystallizes which has a melting point of 140°C. and which is soluble in hydrochloric acid and sodium hydroxide, but not in soda solution. Ferric chloride gives a red color with the substance in hydrochloric acid solution. The compound contains halogen and can be diazo — tized and coupled with R salt to produce a dye. It is apparently a chloro — aminophenol.
One gram of the sulfonic acid from the hydrosulfite reduction is boiled under reflux with 20 cc. 10 per cent hydrochloric acid. After a short time, the solution can be oxidized by air or an oxidizing agent to produce the characteristic red color obtained from 7-amino derivatives of l-amino-8-naphtholsulfonic acids. Since it is known that the Polar dyes contain the toluenesulfonyl group, it may be assumed that the acyl residue connected to the N is the p-toluenesulfonyl group.
The identification of the base is accomplished as follows. Since an aminophenol is formed in the stannous chloride reduction, the original structure was most probably that of an ester or an ether. 8 grams of the base is boiled with 80 cc. 20 per cent hydrochloric acid in a flask fitted with a downward condenser and steam is introduced simultaneously. A lachrymatory liquid distills. It is heavier than water and boils at 175°C. The compound, when warmed with silver nitrate, produces silver chloride. It is oxidized very rapidly by neutral permanganate solution to produce benzoic acid, m. p. 121°. The distillate is’ therefore benzyl chloride.
The liquid remaining in the flask is made alkaline with caustic soda and filtered. It is then acidified and treated with soda solution. In the course of a day, white plates crystallize out. They melt at 139°C. This material is identical with the chloroaminophenol produced in the acid reduction of the dye. It is also identical with the chloroaminophenol derived from 2-nitro-4-chlorophenol. The original base is therefore 4- chloro-2-aminophenylbenzyl ether. This compound can be prepared synthetically by heating l,4-dichloro-2-nitrobenzene with 2.5 moles of 10 per cent sodium hydroxide in an autoclave for 10 hours at 150-160° (see page 109). The resulting sodium salt of nitrochlorophenol is heated for 5 hours with benzyl chloride in alcoholic solution,[76] and the nitro ether is reduced. The base obtained in this way melts at 68°, either alone or mixed with the base from the dye.
By analysis, therefore, Polar brilliant red 3B has the structure:
|
|
Synthesis of the Dye. To a solution of 0.1 mole of H acid in 200 cc. water containing 0.2 mole of soda is added with stirring, at 60-70°C., small portions of toluenesulfonyl chloride until the solution shows no reaction with nitrite. A two — to threefold excess of toluenesulfonyl chloride is required since a toluenesulfonyl group goes onto the hydroxyl group also. When the reaction is complete, enough soda is added to make the mixture a 10 per cent soda solution, and the mixture is boiled for 30 minutes to hydrolyze the toluenesulfonyl group on the —OH. The solution is then cooled in ice and the diazo solution is added. The reaction mixture is stirred for several hours, then warmed to 60° and salted out with a small amount of salt. The dye is purified by reprecipitation. No differences are found between the synthesized product and the original standard, either in dyeing properties or in characteristics in solution.
|
Absorption of Solutions3
a The two bands are not very distinct. |
Monoazo dyes prepared from N-toluenesulfonyl-H acid are, in themselves, not new,100 and similarly, the use of aminophenylbenzyl ethers has previously been disclosed.110 It is to be expected, therefore, that Polar brilliant red 3B could not be patented. One point of scientific interest is the easy splitting of the chloroaminophenylbenzyl ether, merely by boiling with dilute hydrochloric acid, to produce benzyl chloride.111
 22 января, 2016
22 января, 2016  Pokraskin
Pokraskin 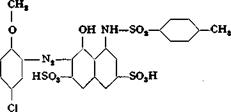
 Опубликовано в рубрике
Опубликовано в рубрике 