This beautiful yellow dye first appeared on the market in 1930. It consists of a yellow powder which dissolves easily in water to give a pure yellow solution. Materials dyed by it are characterized by an extremely pure, almost dazzling, yellow color which cannot be matched by any other previously known yellow dye. The dyed materials exhibit intense fluorescence in ultraviolet light. It seemed probable that the dye was a new compound which would have been patented. In fact, a reference is found in the Fortschritte der Teerfarbenchemie, Vol. 18, p. 867, to German patent 531,291, in which new types of compounds are described as — being very brilliant yellow dyes. The compound contains no azo grouping (not reducible to amines), but does contain nitrogen.
In the case of this dye, it is not necessary to carry out an analysis because soon after its introduction, the dye was mentioned in a small book by Dr. Georg Kranzlein.[77] On page 53 of his book, Dr. Kranzlein says: “An advance in the clarity of tint is seen by comparing brilliant sulfo flavine (Eckert, Hochst, 1929), which is not a triphenylmethane dye, with the purest quinoline yellow available to date.”
Now, German patent 531,291, mentioned above, was, in fact, issued to Dr. Eckert as the inventor, and since the name Eckert, to our knowledge, has not appeared elsewhere in patents describing dyes, we can assume that brilliant sulfo flavine is included in the I. G. patent.
Since the dye is patented, it is probable that it is represented by one of the five examples in the patent. The most likely possibility is example 3, the condensation product from 4-amino-l,8-naphthalic acid anhydride with cyclohexylamine, or example 4, the sulfonation product of this compound.
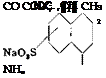 |
CH,
Although the dye is very brilliant, it is not particularly fast to light.
Thus, in this case, a search of the easily available literature has made a tedious analysis unnecessary.
Literature on Analysis of Dyes
1. P. Friedlander (continued by H. E. Fierz-David), Fortschritte der
Teerfarbenfabrikation, Springer, Berlin, 1877-1940.
2. A. Winther, Patente der organischen Chemie, 1877-1905.
3. A. Green, The Analysis of Dyestuffs, 3rd ed., Griffin, London, 1920.
4. G. Schultz, Farbstofftabellen, 7th ed. with Erganzungsband, Akadem.
Verlagsgesellschaft, Leipzig, 1939.
5. Rowe, Colour Index and Supplement, London, 1924.
6. J. Formdnek, Untersuchung und Nachtoeis organischer Farbstoffe
auf spektroskopischem Wege, Springer, Berlin, 1908-1927.
7. A. Brunner, Analyse der Azofarbstoffe, Springer, Berlin, 1929. Addi
tional literature is listed on page 117.
8. H. E. Fierz-David, “The Analysis of Dyestuffs, Yesterday and Today,”
J. Soc. Dyers Colourists, 45,133 (1929).
9. H. E. Fierz-David and M. Matter, “Azo — and Anthraquinoid Dyes
ing the Cyanuric Ring,” J. Soc. Dyers Colourists, 53, 424 (1937).
Since many of the fission products obtained have never been accurately described, the larger handbooks, such as Beilstein, are of little help. On the other hand, it is absolutely necessary to study the available patents carefully, and, if necessary, to perform quantitative, analysis on the products obtained, as pointed out in the introduction to this section. In this connection, reference should be made to the literature compilation given by Fierz and Matter (9, above). Note especially, Forster and Hanson, J. Soc. Dyers Colourists, 42, 272 (1926).
 23 января, 2016
23 января, 2016  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 