Thermochromism is the term used to describe a change of colour as a result of a temperature change. Thermochromic dyes and pigments find application where this colour change is used to indicate a temperature change, for example in plastic strip thermometers, medical thermography and non-destructive testing of engineered articles and electronic circuitry. They may also be used in thermal imaging and for a variety of decorative or novelty effects. Crystal violet lactone (243) is one of the best-known components of thermochromic colorants, and may be used as an example to illustrate the principles. Compound 243 is a non-planar molecule which is colourless. In contact with acid, the lactone ring is opened to give the violet/blue arylcarbonium ion (triarylmethine) structure (244), as illustrated in Scheme 10.2. Similar chemistry is exhibited by certain xanthene derivatives (fluorans), represented by structure 245 in Scheme
10.2, and these compounds enable an extension of the available colour range in the ring-opened forms (246) to orange, red, green and black.
|
Scheme 10.2 Colour formation in the ring-opening of crystal violet lactone and related compounds |
Colour formation reactions of this type are utilised in carbonless copy paper, which is based on the principle of colour formation on the copy as a result of pressure of writing or typing in the master sheet. In such systems, the underside of the master sheet contains the colour former, for example compound 243, encased in microcapsules, which are tiny spheres with a hard polymer outer shell. Pressure on the master sheet breaks the microcapsules and allows the colour former to come into contact with an acidic reagent coated on the copy sheet, thus causing an irreversible colour formation reaction.
Colour formers such as compounds 243 and 245 are not inherently thermochromic. For example, they melt without any change in colour. However, they may be used to generate colour thermally, either irreversibly or reversibly, as composite materials. In thermally sensitive paper, the colour former and an acidic developer, usually a phenol, are dispersed as insoluble particles in a layer of film-forming material. When brought into contact with a thermal head at around 80-120 °C, the composite mixture melts as a result of the localised heating. As a result, the colour former and developer diffuse together and react to form a colour. This process is assisted by the presence of third component, a sensitiser, such as dibenzyl terephthalate, which assists diffusion by acting as a solvent. In this case, the process is irreversible and a permanent image is formed.
Reversible thermochromic systems of this type are also known in which a composite with a similar set of the three ingredients, colour former, developer and solvent (in this case a relatively non-polar solvent such as a fatty acid), are contained together in microcapsules. In these systems, it is curious that, even though essentially the same chemistry is involved, the composite material is usually coloured at low temperatures and decolourises as the temperature is raised. To explain this effect, a mechanism has been tentatively proposed in which it is suggested that at low temperatures the coloured protonated cationic ring-opened form exists as an ion-pair complex with the anion of the phenolic developer. This complex is insoluble in the low polarity solvent at low temperatures. On heating, the complex dissolves, and because of solvation effects associated with the low polarity of the solvent, the complex dissociates to form the colourless neutral ring-closed form and the phenol. On cooling, recrystallisation occurs and this results in the regeneration of the coloured ion-pair complex.
Thermochromism is also shown by certain liquid crystal materials. Chiral nematic liquid crystals adopt a helical structure and, when the pitch length of the helix is of the same order of magnitude as the wavelength of light, colours may be produced from the incident white light by an interference effect. The exact wavelength of the reflected light is dependent on the pitch length. Thermochromism is observed because of the variation in helical pitch length with temperature. The visual effect commonly involves a continuous change through a spectrum of colours as the temperature is raised, and is most striking when viewed against a black background which absorbs the transmitted wavelengths.
 19 января, 2016
19 января, 2016  Pokraskin
Pokraskin 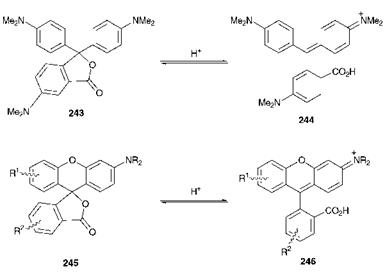
 Опубликовано в рубрике
Опубликовано в рубрике 