Diazotisation, the first stage of azo dye and pigment synthesis, involves the treatment of a primary aromatic amine (ArNH2), which may be carbocyclic or heterocyclic, with nitrous acid to form a diazonium salt (ArN2+Cl~). Nitrous acid, HNO2, is a rather unstable substance that decomposes relatively easily by dissociation into oxides of nitrogen. It is therefore usually generated in the reaction mixture as required by treating sodium nitrite, a stable species, with a strong acid. The mineral acid of choice for many diazotisations is hydrochloric acid. This is because the presence of the chloride ion can exert a catalytic effect on the reaction under appropriate conditions, thus enhancing the reaction rate. Most primary aromatic amines undergo diazotisation with little interference from the presence of other substituents, although they may influence the reaction conditions required. When the reaction conditions are carefully controlled, diazotisation usually proceeds smoothly and in virtually quantitative yield. It is of considerable industrial importance that the reaction may be carried out in water, the reaction solvent of choice for obvious economic and environmental reasons.
Diazotisation is always carried out under strongly acidic conditions, but control of the degree of acidity is of particular importance in ensuring smooth reaction. The overall reaction equation for the diazotisation reaction using sodium nitrite and hydrochloric acid may be given as:
ArNH2 + NaNO2 + 2HCl ArN2+Cl~ + H2O
The reaction stoichiometry therefore requires the use of two moles of acid per mole of amine. However, for a number of reasons, a somewhat greater excess of acid is generally used. One reason is that highly acidic conditions favour the generation from nitrous acid of the reactive nitrosating species which are responsible for the reaction, a feature which will emerge from the discussion of the reaction mechanism later in this chapter. A second reason is that acidic conditions suppress the formation of triazines as side-products which may be formed as a result of N-coupling reactions between the diazonium salts and the aromatic amines from which they are formed. A practical reason for the use of acidic conditions is to convert the insoluble free amine (ArNH2) into its water-soluble proto — nated form (ArNH3+Cl~). However, too strongly acidic conditions are avoided so that the position of the equilibrium is not too far in favour of the protonated amine and allows a reasonable equilibrium concentration of the free amine, which under most conditions is the reactive species as discussion of the reaction mechanism will demonstrate. There is therefore an optimum level of acidity for the diazotisation of a particular aromatic amine, which depends on the basicity of the amine in question. In the case of aniline derivatives, electron-withdrawing groups, such as the nitro group, reduce the basicity of the amino group. As a consequence, for example, the diazotisation of 4-nitroaniline requires much more acidic conditions than aniline itself. Very weakly basic amines, such as 2,4- dinitroaniline, require extremely acidic conditions. They are usually dia- zotised using a solution of sodium nitrite in concentrated sulfuric acid, which forms nitrosyl sulfuric acid (NO + HSO4~). In recent years, heterocyclic aromatic amines, such as aminothiophenes, aminothiazoles and aminobenzothiazoles, have assumed much greater importance as diazo components, particularly in the synthesis of azo disperse dyes. Diazotisation of these amines can prove more problematic. Generally, the use of concentrated acids is required due to the reduction in basicity of the amine by the heterocyclic system and as a result of protonation of heterocyclic nitrogen atoms, and also because the diazonium salts are sensitive to hydrolysis in dilute acids.
It is especially important to control the acidity when aromatic diamines are treated with nitrous acid to form either the mono or bis diazonium salts, a process of some importance in the synthesis of disazo dyes and pigments (see later). p-Phenylenediamine is an example of a
diamine in which either one or both of the amino groups may be dia — zotised by careful selection of reaction conditions. The use of dilute hydrochloric acid can result in smooth formation of the monodiazonium salt. The use of nitrosyl sulfuric acid is required to diazotise the second amino group, since the strong electron-withdrawing effect of the diazo group in the monodiazonium salt reduces the basicity of the amino group that remains.
It is critically important in diazotisation reactions to maintain careful control of the temperature of the reaction medium. The reactions are generally carried out in the temperature range 0-5 °C, necessitating the use of ice-cooling. In some cases, for example with some heterocyclic amines, even lower temperatures are desirable, although temperatures which are too low can cause the reactions to become impracticably slow. Efficient cooling is therefore essential, not least because the reactions are invariably strongly exothermic. One reason for the need for low temperatures is that higher temperatures promote the decomposition of nitrous acid, giving rise to the formation of oxides of nitrogen. The main reason, however, is the instability of diazonium salts. The diazonium cation, although stabilised by resonance, decomposes readily with the evolution of nitrogen, the principal decomposition product being the phenol as illustrated in Scheme 3.3.
|
Scheme 3.3 Thermal decomposition of diazonium salts |
Normally, amines are diazotised using a direct method which involves the addition of sodium nitrite solution to an acidic aqueous solution of the amine. Aromatic amines that also contain sulfonic acid groups, for example sulfanilic acid (4-aminobenzene-1-sulfonic acid), are commonly used in the synthesis of water-soluble azo dyes and in metal salt azo pigments. Because these amines often dissolve with difficulty in aqueous acid, they are commonly diazotised using an indirect method, which involves dissolving the compound in aqueous alkali as the sodium salt of the sulfonic acid, adding the appropriate quantity of sodium nitrite and adding this combined solution with stirring to the dilute acid.
The quantity of sodium nitrite used in diazotisation is usually the equimolar amount required by reaction stoichiometry or in very slight excess. A large excess of nitrite is avoided because of the instability of nitrous acid and since high concentrations can promote diazonium salt decomposition. When direct diazotisation is used, the sodium nitrite is usually added at a controlled rate such that slight excess is maintained throughout the reaction. In practice, this is monitored easily by the characteristic blue colour which nitrous acid gives with starch/potassium iodide paper. When diazotisation is judged to be complete, any remaining nitrous acid excess is destroyed before azo coupling to avoid side-products due to C-nitrosation of the coupling components. This is usually achieved by addition of sulfamic acid, which reacts as follows:
NH2SO3H + HNO2 N2 + H2SO4 + H2O
Because diazonium salts are relatively unstable species, they are almost always prepared in solution as required and used immediately to synthesise an azo dye or pigment. It is generally inadvisable to attempt to isolate diazonium chlorides as they may decompose explosively in the solid state. It is, however, possible to prepare stabilised diazonium salts, which may be handled reasonably safely in the solid state. This is achieved by the use of alternative counter-anions, which are much larger in size and less nucleophilic than the chloride anion. The most commonly used stabilised diazonium salts are tetrafluoroborates (BF4), tetrach — lorozincates (ZnClD and salts obtained from the di-anion of naphthalene-1,5-disulfonic acid. One use of stabilised diazonium salts is in the azoic dyeing of cotton. This process involves the impregnation of cotton fibres with a solution of a coupling component and subsequent treatment with a solution of the stabilised diazonium salt to form an azo pigment, which is trapped mechanically within the cotton fibres. Azoic dyeing, which many years ago was an important method for producing washfast dyeings on cotton, is of relatively limited importance today having largely been superseded by processes such as vat dyeing and reactive dyeing (see Chapter 7).
An outline general mechanism for the diazotisation of an aromatic amine is given in Scheme 3.4. The first stage in the reaction is N — nitrosation of the amine, the nitrosating species being represented in the scheme as Y-N=O. It has been shown that a variety of species may be responsible for nitrosation, depending on the nature of the aromatic amine in question and on the conditions employed for the reaction. Commonly, the nitrosating species may be the nitrosoacidium ion (H2O+-NO), nitrosyl chloride (NOCl), dinitrogen trioxide (N2O3) or the nitrosonium cation (NO+). The formation of each of these species from nitrous acid is illustrated in the series of equilibria shown in Scheme 3.5.
The diazotisation reaction provides a classical example of the application of physical chemistry in the elucidation of the detail of organic
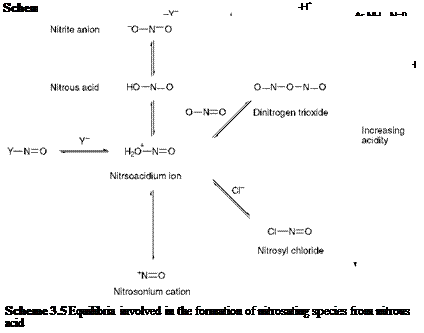
reaction mechanisms. In particular, the results of studies of the kinetics of diazotisation have proved especially informative in establishing the nature of the nitrosating species and the rate-determining step for particular cases. For diazotisation in dilute acids such as sulfuric and perchloric, where the anion is relatively weakly nucleophilic, the nitrosating species has been shown to be dinitrogen trioxide under conditions of low acidity, and the nitrosoacidium ion at higher acidities. In hydrochloric acid, the rate of diazotisation shows a marked increase as a result of catalysis by the chloride anion. The kinetics of the reaction in this case is consistent with a mechanism involving nitrosation of the free amine by reaction with nitrosyl chloride.
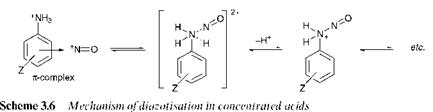
In most of the cases discussed so far, the rate-determining step of the reaction is nitrosation of the free amine. When diazotisation is carried out in concentrated acids, the nitrosonium cation, NO + , is the nitrosating species. In this case, an exchange mechanism has been proposed, as
|
|
illustrated in Scheme 3.6, in which the initial step is reaction of the nitrosonium cation with the protonated amine to form a я-complex. The deprotonation step which follows the exchange becomes rate determining.
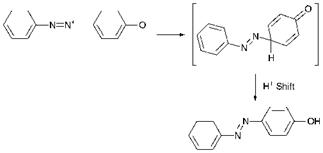
Azo coupling is an example of aromatic electrophilic substitution in which the electrophile is the diazonium cation, ArN2+. Electrophilic substitution reactions, of which nitration, sulfonation and halogenation are arguably the best-known examples, are the most commonly encountered group of reactions of aromatic systems. However, the diazonium cation is a relatively weak electrophile and will therefore only react with aromatic systems which are highly activated to electrophilic attack by the presence of strongly electron-releasing, groups. The most commonly encountered strongly electron-releasing groups are the hydroxy and amino groups, and this in turn means that the most common compounds which are capable of undergoing azo coupling, referred to as coupling components, are either phenols or aromatic amines (either primary, secondary or tertiary). There is a third type of coupling component, commonly a ^-ketoacid derivative, in which coupling takes place at a reactive methylene group. The azo coupling reaction between ben — zenediazonium chloride and phenol is illustrated in Scheme 3.7.
To ensure that the azo dyes and pigments are obtained in high yield and purity, careful control of experimental conditions is essential to minimise the formation of side-products. It is a useful feature of both diazotisation and azo coupling reactions that they may be carried out in water as the reaction solvent. Temperature control, which is so critical in diazotisation reactions, is generally less important in the case of azo coupling. The reactions are normally carried out at or just below ambient temperatures. There is usually little advantage in raising the temperature, other than in a few special cases, since this tends to increase the rate of diazonium salt decomposition more than the rate of coupling.
The experimental factor that requires most careful control in azo
|
Scheme 3.7 The azo coupling reaction between benzenediazonium chloride and phenol |
coupling is pH. There is usually an optimum pH range for a specific azo coupling reaction, which is principally dependent on the particular coupling component used. Phenols are usually coupled under alkaline conditions, in which case the phenol (ArOH) is converted predominantly into the phenolate anion (Ar-O_). There are two reasons why this facilitates the reaction. The first is a practical reason in that the anionic species is more water-soluble than the phenol itself. A second and arguably more important reason is that the — O_ group is more powerfully electronreleasing than the — OH group itself and hence much more strongly activates the system towards electrophilic substitution. Very highly alkaline conditions must be avoided, however, as they promote diazonium salt decomposition. In addition, this can cause conversion of the dia — zonium cation (ArN2+) into the diazotate anion (Ar-N-N-O_), a species which is significantly less reactive than the diazonium cation in azo coupling. Generally, it is desirable to carry out the reaction at the lowest pH at which coupling takes place at a reasonable rate. In the case of aromatic amines as coupling components, weakly acidic to neutral conditions are commonly used. The pH is selected such that the amine is converted substantially into the more water-soluble protonated form (ArNH3+), but at which there is a significant equilibrium concentration of the free amine (ArNH2) which is more reactive to towards azo coupling. Reactive methylene-based coupling components undergo azo coupling via the enolate anion, the concentration of which increases with increasing pH. These compounds are also frequently coupled at weakly acidic to neutral pH values, under which conditions a sufficiently high concentration of enolate anion exists for the reaction to proceed at a reasonable rate and side-reactions due to diazonium salt decomposition are minimised. Commonly, the rate of addition of the diazonium salt solution to the coupling component is controlled carefully to ensure that an excess of diazonium salt is never allowed to build up in the coupling medium, to minimise side-reactions due to diazonium salt decomposition especially when higher pH conditions are required. This is especially important in the synthesis of azo pigments, insoluble compounds from which the removal of impurities is difficult.
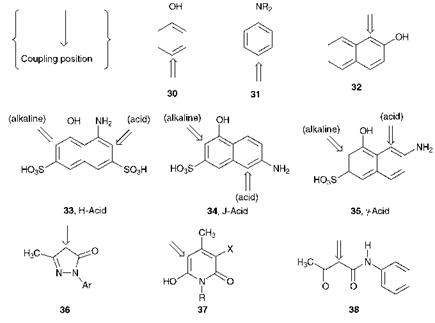
Figure 3.4 illustrates the structures of a range of coupling components commonly used in the synthesis of azo dyes and pigments. In the figure, the position(s) at which azo coupling normally takes place is also indicated. The coupling position is governed by the normal substituent directing effects, both electronic and steric, encountered for aromatic electrophilic substitution. These effects, together with other aspects of the reaction mechanisms involved in electrophilic aromatic substitution are dealt with at length in most organic chemistry textbooks, and so are not considered further here. The coupling components include the relatively simple benzene derivatives, phenol (30) and aniline (31), naphthalene derivatives 32-35, some heterocyclic compounds such as the pyrazolones 36 and pyridones 37, while the ^-keto acid derivatives are exemplified by acetoacetanilide (38). Many coupling components, such as compounds 30-32 and 36-38, are capable of a single azo coupling reaction to give a monoazo colorant. A number of coupling components, for example naphthalene derivatives 33-35, contain an amino and a hydroxy group in separate rings. These compounds are useful because they are capable of
|
Figure 3.4 Structures of some commonly used coupling components |
reacting twice with diazonium salts, thus providing a route to disazo colorants. As an example, 1-amino-8-hydroxynaphthalene-3,6-disulfonic acid, 33, referred to trivially as H-Acid, is used in the synthesis of a number of important azo dyes. The position of azo coupling with this coupling component may be controlled by careful choice of pH. Under alkaline conditions, the hydroxy group is converted into the phenolate anion (-O_) which is more electron releasing than the amino group. In contrast, under weakly acidic conditions it exists un-ionised as the — OH group which is less electron releasing than the amino group. A feature of naphthalene chemistry is that a substituent exerts its maximum electronic effect in the ring to which it is attached, so that under alkaline conditions, azo coupling is directed into the ring containing the hydroxy group, while under weakly acidic conditions reaction takes place in the ring containing the amino group. This selectivity of the coupling position, also shown by coupling components such as J-Acid (34) and y-Acid (35), allows the preparation in a controlled manner of a range of unsymmetrical disazo dyes.
 13 сентября, 2015
13 сентября, 2015  Pokraskin
Pokraskin 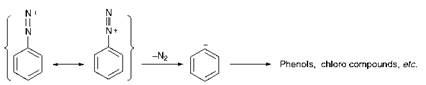
 Опубликовано в рубрике
Опубликовано в рубрике 